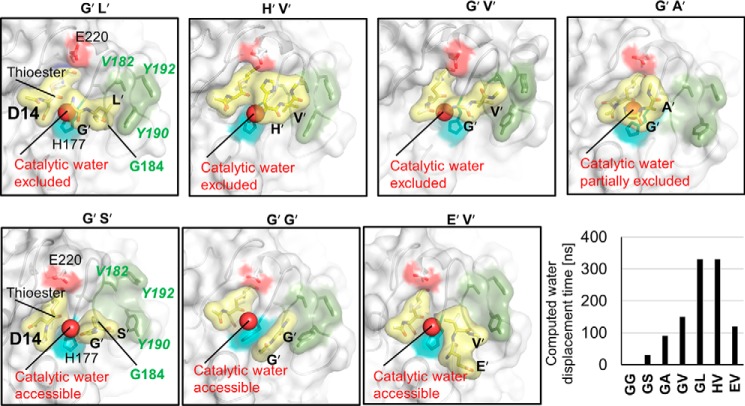
Figure 6.
S2′ pocket of plant legumains has high affinity for hydrophobic P2′ residues, critical for water displacement. All structures are results of 330-ns molecular dynamics simulations with identical starting structures, despite the indicated mutation of the primed residues. The different primed tails are labeled above each structure. Yellow, representation of the still bound product, which is thioester-bound to Cys219 via Asp14; the released primed peptides at the end of the simulation are also indicated. Cyan, catalytic histidine. Red, Glu220. Dark green, S2′ pocket. The putative catalytic water is highlighted. The individual computed water exclusion times are also shown.