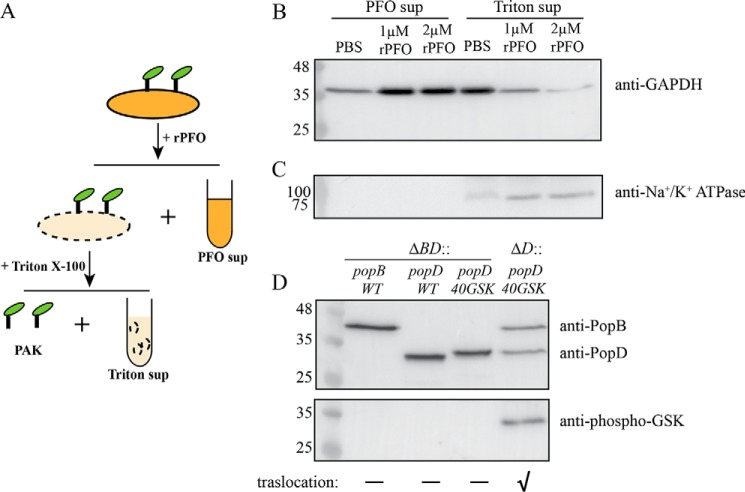
Figure 6.
Insertion of PopD in HeLa cell membranes is promoted by PopB. A, schematic of the membrane protein isolation procedure employed to detect PopD and PopB associated with HeLa cell membranes. Infected cells were first permeabilized with rPFO and centrifuged to separate the released cytosolic components (PFO sup). The pellet containing permeabilized HeLa cells and attached PAK was treated with 0.1% Triton X-100 to selectively solubilize HeLa cell membranes. Insoluble cell debris and bacteria were removed using centrifugation. The supernatant (Triton sup) containing solubilized membrane proteins was precipitated and subjected to immunoblotting as described under “Experimental procedures.” B and C, validation of the membrane protein isolation method. PAKΔexsEΔexoSTYΔpopD::popD-infected HeLa cells were incubated with PBS, 1 μm rPFO, or 2 μm rPFO. PFO sup and Triton sup samples containing 23 μg of total protein were analyzed using immunoblotting for the presence of HeLa cell cytosol or plasma membrane markers using anti-GAPDH (B) and anti-Na+/K+-ATPase (C) antibodies. D, detection of PopD insertion in HeLa cell membranes using a GSK tag phosphorylation assay. PAKΔexsEΔexoSTYΔpopBD or PAKΔexsEΔexoSTYΔpopD strains complemented with pUCP18 plasmid carrying the popB, popD, or GSK-tagged popD genes were incubated with HeLa cells in FBS-free DMEM for 1 h. Proteins associated with HeLa cell membranes were isolated using the membrane protein isolation procedure described in A, and the presence of PopB, PopD, and phospho-GSK PopD was detected by immunoblotting. Representative blots from two independent experiments are shown. The effector translocation ability of used PAK strains into HeLa cells is indicated with a “✓” symbol.