Figure 3. Growing rat musculoskeletal model demonstrating growth restriction with fixed-size implant and growth accommodation with autonomously elongating implant.
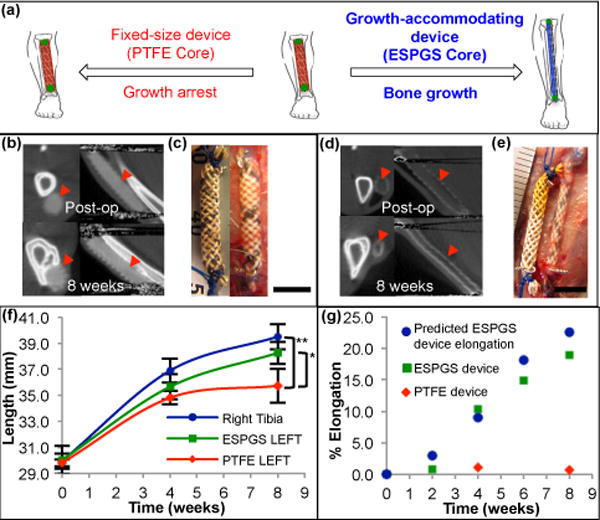
a, Cartoon depicting device implant on the growing tibia. Use of degradable core enables growth accommodation and guided tibial growth (right). Use of nondegradable core results in fixed-size implant and restricted tibial growth (left). b, Micro-CT images in axial (left) & sagittal (right) cross-section show fixed size of PTFE device (
 ) and absence of device elongation over 8-week survival. c, Comparison of fixed-size device at implant (left) and explant (right) shows no significant change in device length (scale bar = 5 mm). d, Micro-CT images of growth-accommodating device show thinning of ESPGS and concurrent lengthening of device (
) and absence of device elongation over 8-week survival. c, Comparison of fixed-size device at implant (left) and explant (right) shows no significant change in device length (scale bar = 5 mm). d, Micro-CT images of growth-accommodating device show thinning of ESPGS and concurrent lengthening of device (
 ) over 8-week survival. e, Comparison of growth-accommodating device at implant (left) and explant (right) shows significant device elongation (scale bar = 5 mm). f, Tibial growth. Implantation of growth-accommodating device (ESPGS core) versus fixed-size device (PTFE core) led to distinct growth profiles (mean ± s.d.). Fixed-size implant caused progressive growth restriction and ultimately growth arrest in the final 4 weeks (
) over 8-week survival. e, Comparison of growth-accommodating device at implant (left) and explant (right) shows significant device elongation (scale bar = 5 mm). f, Tibial growth. Implantation of growth-accommodating device (ESPGS core) versus fixed-size device (PTFE core) led to distinct growth profiles (mean ± s.d.). Fixed-size implant caused progressive growth restriction and ultimately growth arrest in the final 4 weeks (
 ). Growth-accommodating implant provided mild growth restriction during first 4 weeks, but permitted physiologic bone growth in last 4 weeks (
). Growth-accommodating implant provided mild growth restriction during first 4 weeks, but permitted physiologic bone growth in last 4 weeks (
 ). By 8 weeks, left tibial length with fixed-size implant was statistically less than left tibial length with growth-accommodating implant and right tibial length (
). By 8 weeks, left tibial length with fixed-size implant was statistically less than left tibial length with growth-accommodating implant and right tibial length (
 ) (* P = 0.037, ** P = 0.004, one-way ANOVA post-hoc Tukey test). (n = 3 animals per group) g, Comparison of predicted and observed device elongation in representative animals. Observed ESPGS device elongation (
) (* P = 0.037, ** P = 0.004, one-way ANOVA post-hoc Tukey test). (n = 3 animals per group) g, Comparison of predicted and observed device elongation in representative animals. Observed ESPGS device elongation (
 ) closely correlated with predicted elongation (
) closely correlated with predicted elongation (
 ). Fixed-size implant (
). Fixed-size implant (
 ) shown for comparison.
) shown for comparison.
