Figure 4. Growing piglet heart valve proof-of-concept - exploring use of growth-accommodating annuloplasty device in a dynamic cardiovascular environment.
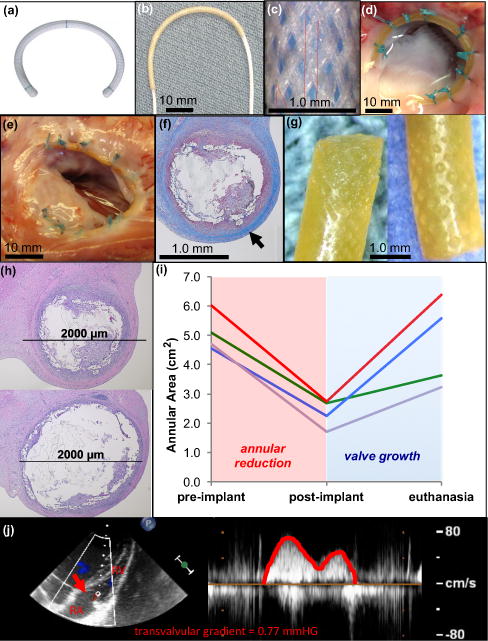
a, Commercially available fixed-size annuloplasty device used in adults. b, UHMWPE biaxially braided sleeve placed over pre-curved cylindrical ESPGS polymer core. c, Biaxially braided sleeve pattern. d, Ex vivo demonstration of ring implantation with en face view of tricuspid valve. Growth-accommodating ring is secured to valve annulus with conventional suturing technique used for ring implantation in adults. UHWPE braided sleeve ends and body of device are secured to annulus. e, En face view of freshly explanted tricuspid valve and ring 12 weeks after surgery. Ring is intact and integrated into annular tissue without evidence of thrombus formation or dehiscence. f, Cross-section through explanted ring demonstrating collagen layer that grew over device (
 ). g, Explanted ESPGS samples demonstrate erosion at polymer surface. h, Cross-sections through segments of ring/annulus demonstrate regions of significant core erosion with ring thinning (top) and areas of less significant core erosion with less thinning of ring (bottom). i, Tricuspid valve area at three study points: pre-implant, post-implant, and euthanasia. All animals experienced valve reduction following ring implantation (red region), and experienced valve growth in the post-operative period (blue region). (GREEN line: 5 week animal, RED line: 12 week animal, BLUE line: 16 week animal, PURPLE line: 20 week animal) j, Continuous wave color Doppler echocardiogram demonstrates only trivial gradient (0.77 mmHg) across tricuspid valve orifice (
). g, Explanted ESPGS samples demonstrate erosion at polymer surface. h, Cross-sections through segments of ring/annulus demonstrate regions of significant core erosion with ring thinning (top) and areas of less significant core erosion with less thinning of ring (bottom). i, Tricuspid valve area at three study points: pre-implant, post-implant, and euthanasia. All animals experienced valve reduction following ring implantation (red region), and experienced valve growth in the post-operative period (blue region). (GREEN line: 5 week animal, RED line: 12 week animal, BLUE line: 16 week animal, PURPLE line: 20 week animal) j, Continuous wave color Doppler echocardiogram demonstrates only trivial gradient (0.77 mmHg) across tricuspid valve orifice (
 ) at time of euthanasia, indicating that growth-accommodating ring did not induce valve stenosis after implantation. RA = right atrium, RV = right ventricle.
) at time of euthanasia, indicating that growth-accommodating ring did not induce valve stenosis after implantation. RA = right atrium, RV = right ventricle.
