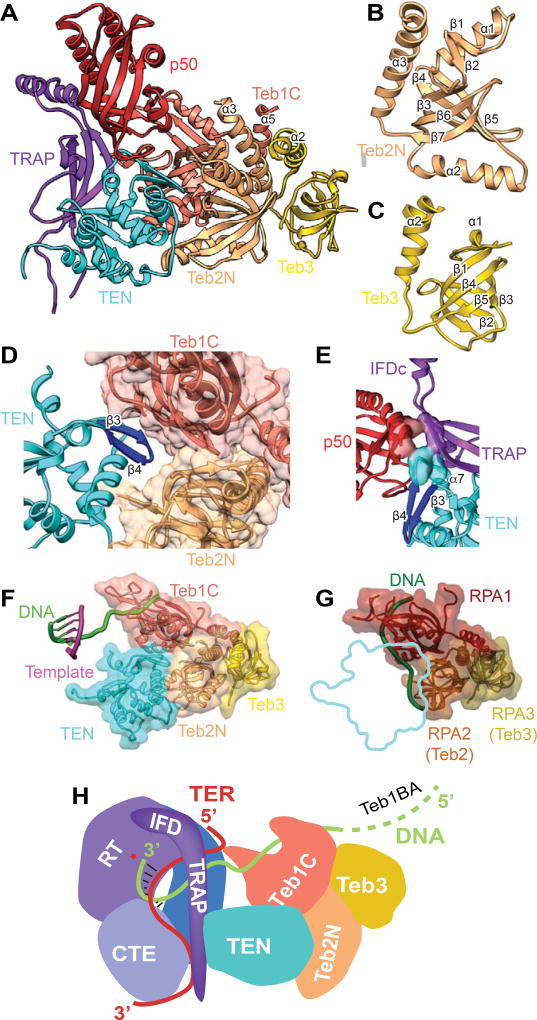
Figure 6. Structures and Interaction of p50 and TEB with TERT.
(A) Structure of IFD-TRAP-TEN-p50-TEB complex. TEN interacts with TRAP, p50, Teb1C, and Teb2N. TRAP interacts with p50 and TEN. Teb1C α5, Teb2N α3, and Teb3 α2 form a three-helix bundle. Teb1C structure is same as the crystal structure (PDB 3U50) except for the presence of α5 and the structure of the Zn ribbon motif (Figure 5C). The secondary structure of p50, modeled de novo, is a 6-stranded β-barrel with 4 α-helices.
(B) Structure of Teb2N/Rpa2N modeled from the cryoEM map. The structure is a 5-stranded β-barrel with 3 α-helices.
(C) Structure of Teb3/Rpa3 modeled from the cryoEM map. The structure is a 5-stranded β-barrel with 2 α-helices.
(D) Zoomed view of binding interface between Teb1C, Teb2N, and TEN. The TEN β3-β4 loop inserts between Teb1C and Teb2N.
(E) Close up view of the three-way interactions between p50, IFD-TRAP, and TEN, with interacting residues shown as surface. P50 and TRAP interact with TEN β4-α7 loop and α7, and p50 interacts with IFD-TRAP at the corner of the L linking them.
(F) TEN-TEB complex with sstDNA bound on Teb1C. The position of the sstDNA on Teb1C and the template-DNA duplex is shown relative to the TEN-TEB complex.
(G) RPA complex with DNA bound (PDB 4GNX) on RPA1 and RPA2 (equivalent to Teb2/Rpa2), with outline of TEN domain interaction in TEN-TEB complex to illustrate that TEN would occlude DNA binding surface on Teb2/Rpa2 in the Tetrahymena RPA complex.
(H) Cartoon illustrating the path of TRE-template-TBE on TERT and sstDNA from the active site of TERT to TEB. p50 is omitted for clarity.
See also Figures S4.