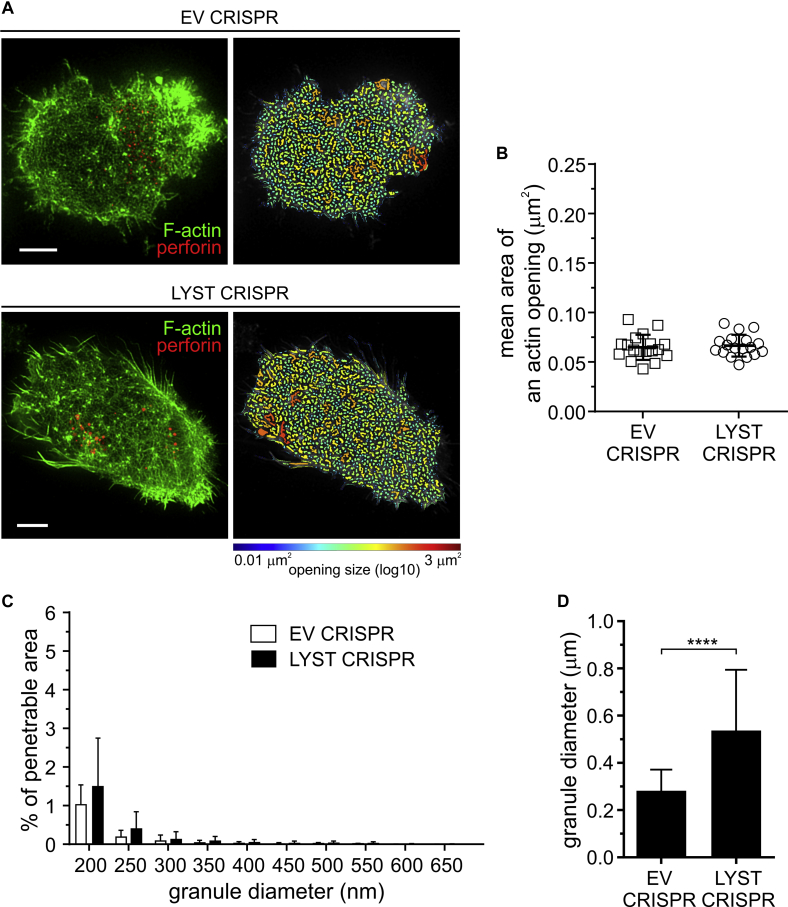
Fig E4.
Membrane-proximal actin meshwork at synapses formed through engagement of lymphocyte function–associated antigen 1 (LFA-1). A, EV or LYST CRISPR NK92mi cells were incubated on cover slips coated with ICAM-1, stained with anti-perforin antibody (red) and phalloidin (green), and visualized by using STED microscopy. First column, Super-resolution images of F-actin meshwork and lytic granules proximal to the plasma membrane. Scale bars = 5 μm. Second column, Openings among F-actin at the synapse shown as heat maps; the smallest holes (0.01 μm2) are indicated in blue, and the largest (approximately 3 μm2) are indicated in red. B, Quantification of the average size of actin meshwork openings at the synapse center for the indicated NK92mi cells stimulated as in Fig E4, A. The graph represents means ± SDs (n = 18 cells per condition from 3 independent experiments). C, Quantification of the proportion of the synapse area predicted to be penetrable by vesicles of 200 to 800 nm in diameter for the same cells as in Fig E4, B. The graph shown means + SDs (n = 18 cells per condition from 3 independent experiments). There was no significant change in the mean area of actin openings or the percentage of actin meshwork permeability between EV and LYST CRIRPS cells. D, Size of lytic granules. EV or LYST CRISPR NK92mi cells were labeled and visualized as in Fig E4, A, and the perforin-positive vesicles were recorded in 3 dimensions (x-y-z plane). Bar graph shows the average granule diameter in the indicated cells; error bars indicate SDs. ****P < .0001, unpaired t test.