Fig. 19.
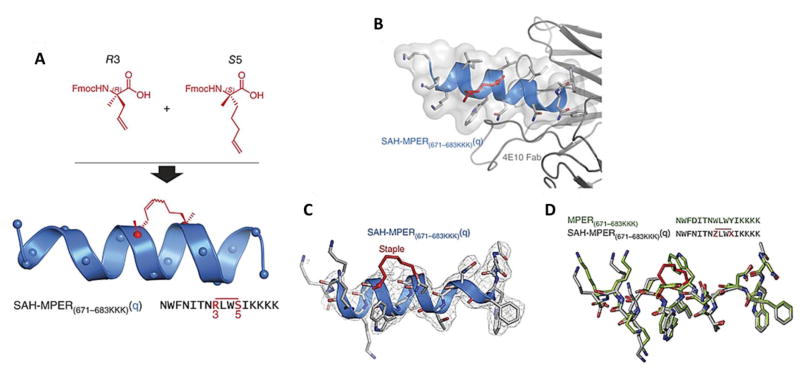
(A) Chemical optimization of i, i + 3 hydrocarbon stapling: Top, design and synthesis of SAH-MPER(671–683KKK)(q), in which the N-terminal S5 residue was replaced with R3 to lead to efficient i, i + 3 olefin metathesis under standard reaction conditions. (B) Crystal structure of SAH-MPER(671–683KKK)(q) (shown as a blue ribbon and gray transparent van der Waals surface) bound to 4E10 Fab, at 2.9-Å resolution. (C) 2Fo–Fc electron density map (1σ level) of the antibody-bound SAH-MPER(671–683KKK)(q) peptide. (D) Superposition of the native (green; PDB 2FX7)18 and i, i + 3–stapled (gray) MPER(671–683KKK) peptides, highlighting the similarity of antibody-bound structures, aside from the appended C-terminal lysines and the incorporated staple. Z and X represent R3 and S5, respectively, in the staple (red bar above sequence). Reprinted from [231].