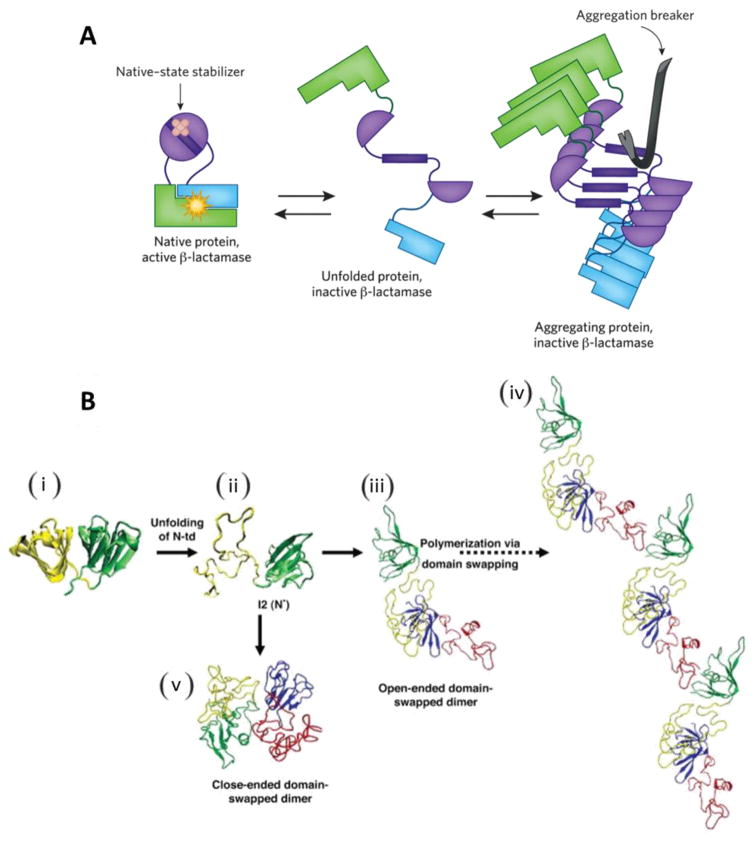
Fig. 9.
(A) Functioning of a chemical chaperone: β-lactamase function is restored when aggregation of the target protein is inhibited. This can occur either through stabilization of the native structure (left) or through inhibition of the process of amyloid self-assembly (right). Reprinted from [86]. (B) Schematic summary of human γD-crystallin (a member of crystallin families) polymerization. (i) Crystal structure of human γD-crystallin. (ii) Simulated monomeric aggregation precursor (I2), often referred as N* in the general mechanism of protein aggregation in literature. (iii) Simulated structure of open-ended domain-swapped dimer. (iv) Simulated structure of close-ended domain-swapped dimer. (v) Model of human γD-crystallin hexamer formed via domain swapping. Reprinted from [84].