Fig. 6.
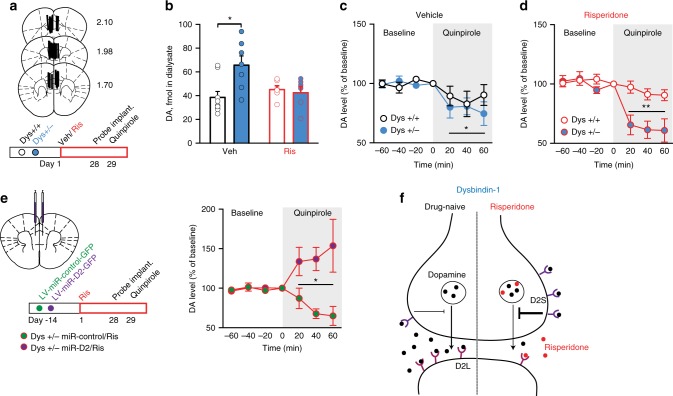
Genetic variations reducing dysbindin-1 confer unique potentiation of cortical D2 autoreceptor activity following antipsychotics. a Localization of probe dialyzing portion within the mPFC and timeline of the experiment. Dys+/+ and +/− mice received chronic vehicle or risperidone treatment and, after 4 weeks, were implanted with a dialysis probe for measurement of basal extracellular dopamine levels and quinpirole-induced dopamine release. b Increased basal extracellular dopamine levels in the mPFC of Dys+/− are restored by risperidone (two-way ANOVA, genotype × treatment, F(1, 27) = 4,61, p < 0.05; n = 6–7/group). p = 0.39 vs. Dys+/+. c Quinpirole infusion (gray area) equally decreased extracellular dopamine release in the mPFC of Dys+/+ (white circles) and +/− (blue circles) mice after chronic treatment with Veh (two-way RM ANOVA, time, F(6, 60) = 2,83; p < 0.05). *p < 0.05 vs. baseline. d Dys+/− (blue circles) mice following chronic treatment with Ris (in red) showed higher efficacy of mPFC quinpirole infusion (gray area) in reducing extracellular dopamine release (two-way RM ANOVA, time × genotype, F(6, 66) = 5.76, p < 0.0005). Quinpirole had no effect on risperidone-treated +/+ mice (white circles; p = 0.38 vs. baseline). **p < 0.005 vs. Dys+/+. e Dys+/− mice received a synthetic microRNA (miR) to inactivate D2 receptors (LV-miR-D2, green circles) or a control miR (LV-miR-control, purple circles) in the mPFC. After 2 weeks, animals received daily injections of Veh or Ris for 28 days (to parallel behavioral experiment in Figs. 2, 4) and then have been implanted with a dialysis probe. On the following day in vivo microdialysis quinpirole-induced dopamine release was measured. In Dys+/− treated with Ris, D2 silencing even increased dopamine release. Injection of a control miR in Dys+/− Ris-treated mice further confirmed a decrease of quinpirole-mediated dopamine release (two-way RM ANOVA, time × group effect, F(12, 78) = 4.05, p < 0.0005; n = 5–7 each group). Error bars represent S.E.M. *p < 0.05, **p < 0.005. f Figure model. In basal drug-naive conditions, genetic variations resulting in reduced dysbindin-1 expression increases tonic extracellular dopamine levels (black dots). Long-term administration of risperidone (red dots) alters the balance between short and long isoforms of D2 receptors, resulting in the potentiation of D2 presynaptic signaling. Antipsychotic drugs accumulate in synaptic vesicles and are secreted from upon exocytosis49. Moreover, antipsychotic drugs preferentially bind D2L postsynaptic receptors47, 63, which might cause D2L/D2S imbalance in favor of D2S autoreceptors13