Fig. 6.
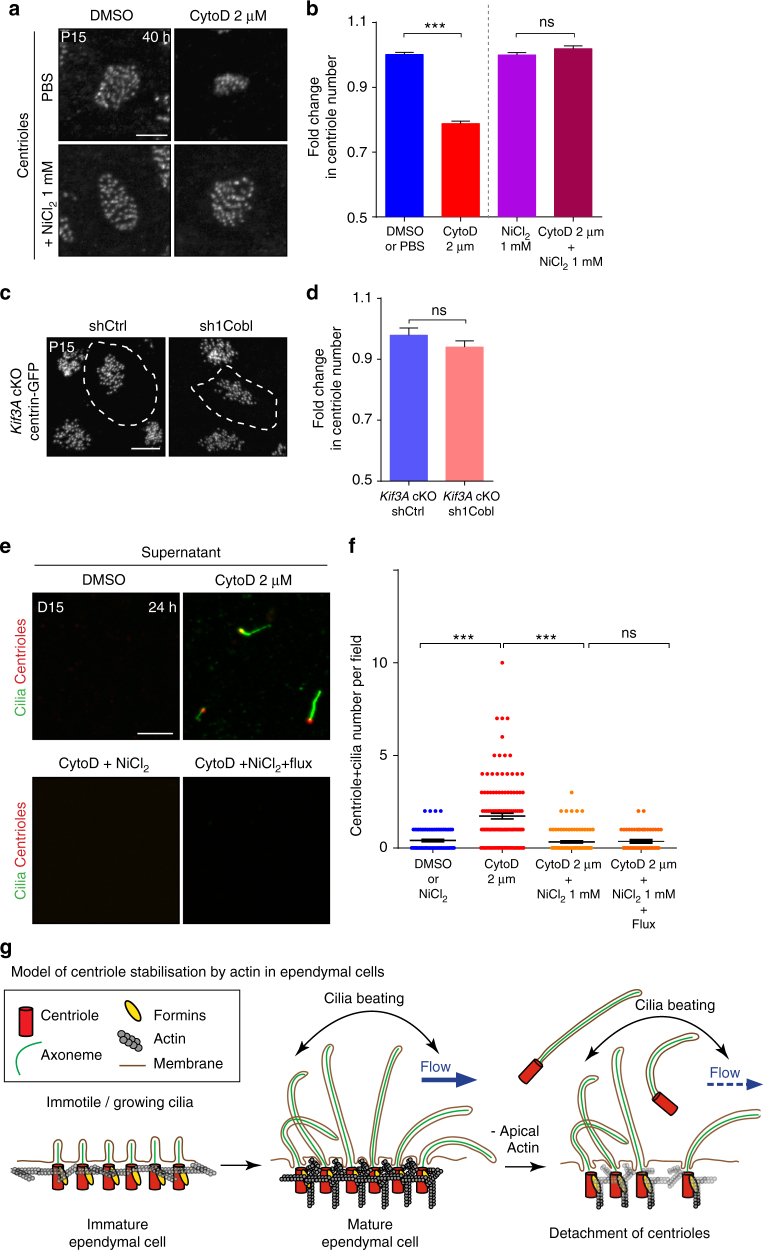
Forces generated by cilia beating cause centrioles/cilia to detach from actin-deficient ependymal cells. a, b Mature (P15) ependymal cells from explants treated for 40 h with PBS or DMSO (Control), 1 mM NiCl2 to block cilia beating, 2 µM cytochalasin-D (cytoD) to depolymerise actin, or with both drugs; a γ-tubulin stained centrioles. b Fold change in centriole number relative to DMSO- or NiCl2-treated contralateral brain, showing that cilia beating induces centriole destabilisation in absence of actin; P-values were determined by one-way ANOVA followed by Dunn’s multiple comparison test. n = 781 cells for control-, 597 for CytoD-, 819 for NiCl2- and 547 for CytoD + NiCl2-treated explants, from three independent experiments; ***P ≤ 0.0001; ns P > 0.05. c, d Lateral walls of Centrin2-GFP ciliary mutant mice (Kif3A cKO) electroporated at birth with shCtrl or sh1Cobl and a reporter construct (represented as white dashed lines) and fixed at P15. c Representative Centrin2-GFP+ centriolar patches. d, Fold change in centriole number relative to surrounding cells, showing no effect of Cobl-depletion on centriole number in ciliary mutant mice. P-values were determined by the Mann–Whitney test. ns, P > 0.05. n = 101 electroporated cells for Kif3A-cKO shCtrl and Kif3A-cKO sh1Cobl from three independent experiments. e Representative images of the supernatant of mature ependymal cells from Centrin2-GFP mice in culture. After 15 days of the onset of differentiation, cells were treated for 24 h with cytochalasin-D or DMSO with or without 1 mM NiCl2 and an external fluid flow; isolated centrioles (Centrin2-GFP, red) with a cilium (GT335, green) were observed in the supernatant of CytoD-treated cultures. f Number of centrioles associated with a cilium per 63 × field. n = 76 fields for DMSO-, 112 for CytoD-, 71 for CytoD + NiCl2- and 61 for CytoD + NiCl2 + Flux-treated cell cultures, from three independent experiments; P-values were determined with the Mann–Whitney test; ***P ≤ 0.0001. Scale bars=5 μm. g Working model: Ependymal cilia beating leads to actin enrichment at the centriolar patch. Apical actin is in turn crucial for stabilising centrioles exposed to cilia beating and fluid flow forces. In cells with impaired apical actin, beating cilia cause centrioles and their associated cilia to detach from the cells. Error bars represent the sem in all graphs