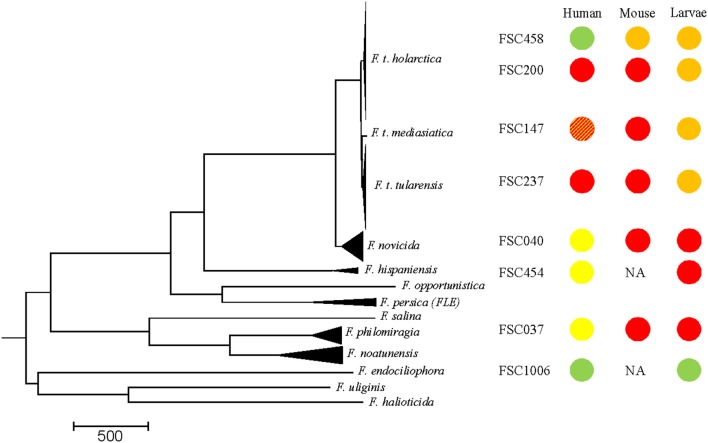
Figure 4.
Comparison between data of Francisella spp. lethality in G. mellonella generated in this study and previously published data on Francisella spp. lethality in mice by subcutaneous or intracutaneous inoculation (Bell et al., 1955; Owen et al., 1964; Guryčová, 1998; Kieffer et al., 2003; Salomonsson et al., 2009; Molins et al., 2014; Propst et al., 2016; Timofeev et al., 2017) and severity of disease in humans (Saslaw et al., 1961a,b; Dienst, 1963; Kugeler et al., 2009). Lethality of the different Francisella strains in G. mellonella (larvae death within 3 days, infectious dose 106 bacteria/mL) and mice (LD50 < 103) was scored as high lethality (red), intermediate lethality (larvae death > 3 days, LD50 mice > 103) (orange) and non-lethal (green). Severity of disease caused by the Francisella strains in humans, was scored as incapacitating disease in humans (red), causing disease only in humans with a compromised immune response or underlying health defects (yellow) and non-virulent (green). There is only limited data on the virulence of F. t. mediasiatica in humans (Timofeev et al., 2017) (striped) and data on F. hispaniensis and F. endociliophora lethality in mice are lacking. The LVS strain is a live attenuated strain that was selected for vaccination in humans but still remains unlicensed for human use (Sandström, 1994). NA, data not available.