Abstract
Total knee arthroplasty (TKA) is highly associated with post-operative pain. The present randomized trial aimed to explore the possible post-operative pain management by a different combination of analgesics or opioids (ketamine and bupivacaine) following TKA. A total of 84 patients were randomly divided into four groups. All subjects were anesthetized for TKA surgery and received post-operative pain management via intra-articular saline (control group; n=23), ketamine (2 mg/kg) infused with saline (ket group; n=21) bupivacaine (0.5 mg/kg) infused with saline (bupi group; n=20) or ketamine (2 mg/kg)+bupivacaine (0.5 mg/kg) infused with saline (ket+bupi group; n=20) at the end of the surgery. Additional, post-operative analgesia was infused with the aid of patient-controlled analgesia with morphine. A reduction in the levels of pain score (verbal rating scale and visual analog scale), opioid consumption, time of ambulation, hospital stay and adverse events were observed in the ket+bupi group compared with the other groups. Meanwhile, the satisfaction score and knee flexion degree were improved following treatment with the ket+bupi regimen. Therefore, the multimodal analgesic regimen (ket+bupi) may be useful in mitigating post-operative pain as and improving knee mobilization following TKA.
Keywords: total knee arthroplasty, ketamine, bupivacaine, morphine, verbal rating scale, visual analog scale
Introduction
Total knee arthroplasty (TKA) is the standard surgical procedure performed globally for patients with end-stage osteoarthritis (OA) or rheumatoid arthritis (RA) (1). Pain management or control following TKA is a crucial factor for the successful outcome of knee surgery as poorly controlled pain following TKA may lead to increased anxiety, morbidity or psychological disorders, prolong the overall recovery or healing process, and increased readmission rate (2,3). Several steps have been taken to control or manage post-operative pain management; however, 20–25% of patients who have undergone TKA still experience severe to moderate pain at 1 year following the procedure (chronic pain), which may be due to acute severe pain (inadequate post-operative pain management) that leads to chronic pain for many years (4,5).
Therefore, controlling or managing post-operative pain following TKA may considerably improve quality of life by enhancing motility, reduce hospitalized days with fewer pulmonary or cardiac complications, and decrease morbidity and mortality rate (6). Currently, the usage of epidural analgesia, femoral nerve block, and cyclooxygenase-2 inhibitors are recommended for post-operative pain management following TKA. However, these drugs result in several adverse events such as nausea/vomiting, constipation, sedation, pruritus, and depression (7–9). A number of previous studies have indicated that a multimodal pain management strategy with femoral nerve block (combining different analgesic agents-holistic activity) are successfully used to manage post-operative pain following TKA owing to its opioid sparing activity (10,11). Among those analgesics morphine, ketamine, bupivacaine, acetaminophen, clonidine and tramadol are commonly prescribed (12–14).
Ketamine is a potent analgesic (used as general or local anesthesia) which can interact with several types of receptors (as an antagonist), including adrenergic, serotonergic, muscarinic, opioids, and N-methyl D-aspartate (NMDA) (15). Hence, it is used for the management of pre-, intra- and postoperative pain (16). Adam et al (17) previously reported that small doses of ketamine (0.5 mg/kg) may improve knee mobilization following TKA; whereas bupivacaine is typically used for intra-articular (IA) analgesia because of its extended period of effectiveness (4–7 h) or long-lasting anesthetic properties (18). Furthermore, ketamine has been reported to be more efficient when combined with bupivacaine and a femoral nerve block (19). Bupivacaine is recommended for treating post-operative pain management following TKA, as it induces fewer adverse effects and does not affect any motor function (4,20). Previously, Inanoglu et al (21) concluded that ketamine and bupivacaine (multimodal regimen) could effectively control the post-operative pain following tonsillectomy. Furthermore, Batra et al (19) in their pilot study indicated that ketamine and bupivacaine could provide better pain relief following arthroscopic knee surgery by evaluating visual analog scale (VAS) and opioids consumption in arthroscopic knee surgery. Based on the above evidence (19), the present randomized, double-blind, controlled study was designed to evaluate the potential post-operative pain control with different combination of opioids following TKA with respect to VAS/verbal rating scale (VRS), satisfactory score, knee flexion, ambulatory time, opioid consumption rate and adverse effects.
Materials and methods
Participant enrolment
The present randomized, double-blind, single center, retrospective clinical trial was conducted at Hanzhong Hospital of Traditional Chinese Medicine (Hanzhong, China) and approved by the Scientific Ethics Committee (HH-TCM-03-2016 dated on March 2, 2016) of Hanzhong Hospital of Traditional Chinese Medicine (Hanzhong, China) according to the guidelines of the Declaration of Helsinki. A total of 105 patients [ASA I–III grade (5); aged between 50–60; 42 males and 63] who were scheduled to undergo Unilateral TKA between July 2016 and August 2017 were initially enrolled in the present trial. Inclusion criteria were as follows: Adult undergoing unilateral TKA, diagnosed with degenerative end-stage OA of the knee (score of >2 on Kellgren-Lawrence scale) (22). Exclusion criteria were as follows: Bilateral TKA, RA, <18 or >80 years, previous history of knee arthroplasty or chronic treatment with opioids (opioids tolerance), allergic to amide or sulpha anesthetics, unable to understand the VAS and VRS, exhibiting major hepatic, cardiac or renal disorders. Based on these criteria, 84 eligible patients were enrolled, once all details about the trail were explained and patients had provided written informed consent. Furthermore, during the pre-anesthetic visit, the subjects were taught about the VAS and VRS in detail.
TKA surgical procedure
All patients were fasted for 12 h and received 0.01 mg/kg intramuscular atropine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as premedication and their vitals were checked prior to surgery. Prior to surgery, all patients received fentanyl [2 mg/kg; intravenously (i.v)], Propofol (3 mg/kg; i.v) and ropivacaine HCL (0.75%; i.v catheter)-femoral nerve block (all obtained from Sigma-Aldrich; Merck KGaA). Anesthesia was maintained with 60% nitrous oxide in oxygen and sevoflurane. Patients were also administered intra-operative analgesic (morphine, 0.2 mg/kg; oxycodone, 0.1 mg/kg; fentanyl, 0.2 mg/kg; acetaminophen 0.2 mg/kg; Sigma-Aldrich; Merck KGaA) during TKA surgical procedure. Finally, a single dose of IA saline, ketamine, bupivacaine or ketamine and bupivacaine (20 ml) using a spinal needle between the deep and superficial soft tissue of the knee at the end of the surgery, as indicated below. TKA surgery was performed by an experienced surgeon with technical assistance and anesthesiologist.
All patients were then transferred to the post-operative care unit. The continuous femoral nerve block (ropivacaine 0.5%, 0.1 ml/kg/h) was maintained for 24 h, followed by infusion of Ketorolac (30 mg; i.v; Sigma-Aldrich; Merck KGaA) every 6 h for 24 h. Post-operative analgesia (administered with the aid of patient-controlled analgesia) comprised of morphine (bolus of 1 mg; 5 min lockout period) for 48 h. Immediately following the surgical procedure, patients were asked to commence physiotherapy training, with the help of a physiotherapist until discharge. Continuous infusion of pain regimen and femoral nerve block were discontinued at 48 h following admission to the post-operative care unit, and was replaced with a number of oral medications (oxycodone, tramadol, ibuprofen and acetaminophen are supplied by Sigma-Aldrich; Merck KGaA) until pain subsided. Following 48 h in the post-operative care unit, patients were transferred to a rehabilitation center for further physiotherapy training. Demographic data including age, sex, height, weight, ASA status and duration of surgery were recorded for each patient. A flow chart of the present methodology is presented in Fig. 1.
Figure 1.
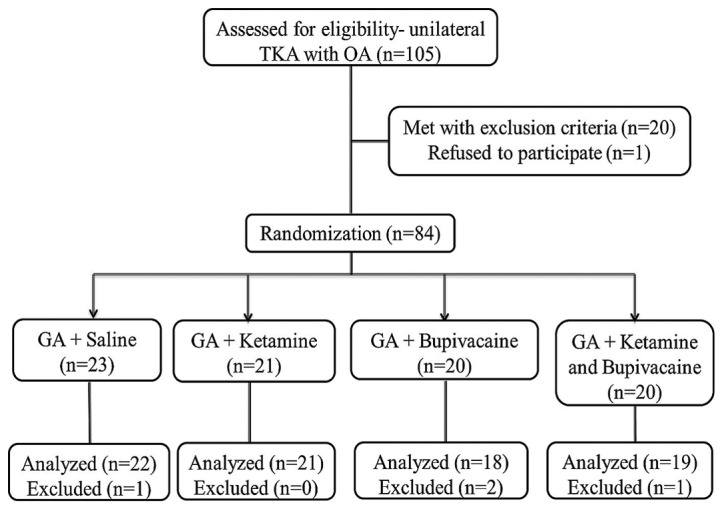
A flowchart of the present trial. TKA, total knee arthroscopy; OA, osteoarthritis; GA, general anesthesia.
Grouping
A total of 84 eligible patients (28 male and 56 female) aged 42–74 years were chosen and randomly separated into four groups using a computer-generated randomization program (REDcap; version 8; National Institutes of Health, Bethesda MD, USA) by an independent statistician. Patients were anesthetized with general anesthesia (as indicated above) followed by IA injection of 20 ml saline (control group; n=23), ketamine (2 mg/kg) infused with saline (ket group; n=21), bupivacaine (0.5 mg/kg) infused with saline (bupi group; n=20), or ketamine (2 mg/kg)+bupivacaine (0.5 mg/kg) infused with saline (ket+bupi group; n=20) using spinal needle between the deep and superficial soft tissue of knee at the end of the surgery. All study opioids were supplied as coded and blinded from both the surgeon and anesthesiologist.
Measurement of various parameters
The opioid consumption of all experimental groups was measured by converting to morphine equivalent using conversion factors as indicated by Surdam et al (23). The VRS was determined verbally by the patient as a self-rated pain score, which corresponded to the degree of pain at rest (19). The VRS ranged from 0–5, where 0 represented no pain, 1 represented mild pain, 2 represented moderate pain, 3 represented severe pain, 4 represented very severe pain and 5 represented excruciating, or unbearable pain. VAS consisted of a horizontal line 100 mm in length, with numerical endpoints of 0 (no pain) and 100 (intolerable or worst pain). Patients were asked to mark the line at the level according to their pain intensity at rest (24). Patients satisfactory score were evaluated using a Likert scale which ranges from 0–3, where 0 represented dissatisfied, 1 represented least satisfied, 2 represented partially satisfied and 3 represented fully satisfied (25). Knee flexion or extension (degree) were measured using a flexible electro-goniometry on the 2nd day (48 h following admission to the post-operative care unit) using the method described by Matassi et al (26).
The time of ambulation (h) and the length of hospitalization (days; including post-operative and rehabilitative periods) for each patient were also noted. Adverse events including sweating, sedation, dizziness, urinary retention, constipation and post-operative nausea and vomiting were recorded. All parameters were measured or recorded at 48 h as the present study focused on acute post-operative pain management (excluding ambulation and length of hospitalization). In case of total opioid consumption, VRS, VAS, and satisfactory score were determined at 0, 2, 4, 8, 12, 24 and 48 h by a registered nurse anesthetist and anesthesiologist. All patients, and the surgeon, nurse and statistician were blinded to the interventions used.
Statistical analysis
Data are presented as the mean ± standard deviation. VRS and VAS rating and demographic data were analyzed and compared using χ2 test. Level of opioid consumption, satisfactory score, adverse events, ambulation, hospital stay duration and flexion angle data were compared between the experimental groups using one-way analysis of variance followed by post hoc multi-comparison Dunnett's test. SPSS (version 21; IBM Corp., Armonk, NY, USA) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic data
A total of 82 patients completed the study, the remaining 2 patients (1 each from the ket and bupi groups) were excluded due to femoral nerve block failure. Demographic data (age, sex, height, weight, ASA status and duration of surgery) of patients in the experimental groups are presented in Table I. At baseline, the values of all demographic data did not show any significant difference among groups.
Table I.
Demographic data of patients in each experimental group.
| Parameters | Saline+GA (n=23) | Ketamine+GA (n=21) | Bupivacaine+GA (n=20) | Ket+bupi+GA (n=20) |
|---|---|---|---|---|
| Sex (male/female) | 7/16 | 6/15 | 8/12 | 7/13 |
| ASA status (I/II/III) | 5/13/5 | 6/9/6 | 8/7/5 | 6/9/5 |
| Height, cm | 161.42±21.04 | 162.44±20.10 | 160.34±17.34 | 162.33±23.73 |
| Weight, kg | 71.44±13.30 | 75.43±12.02 | 73.64±12.93 | 76.23±10.45 |
| Age, years | 54.12±7.11 | 55.78±9.34 | 57.11±10.11 | 56.77±9.77 |
| Duration of surgery, min | 124.57±11.34 | 119.43±30.95 | 121.32±28.37 | 119.32±28.37 |
Data are presented as the mean ± standard deviation unless otherwise stated. GA, general anesthesia; Ket+bupi, ketamine and bupivacaine.
Total opioids consumption
Fig. 2 presents the total opioid consumption rate (morphine equivalence) of patients in each experimental group. Total opioid consumption during the 48 h stay in post-operative care was significantly decreased (P<0.01) in the ket+bupi combination group (25.42 mg) as compared with the control (32.5 mg) group. Compared with the control group (32.5 mg) ket (28.44 mg) and bupivacaine (27.8 mg) significantly lowered (P<0.05) the opioid consumption.
Figure 2.
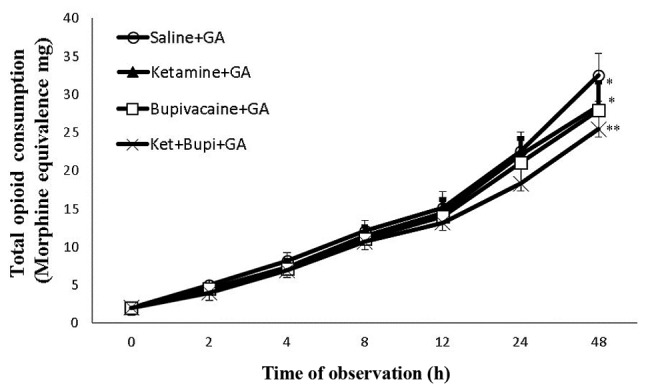
The total opioid consumption rate (morphine equivalence) of patients in each experimental group. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 vs. saline+GA group. GA, general anesthesia; Ket+Bupi, ketamine and bupivacaine.
Pain score
The pain intensity of each patient was measured in the form of VAS and VRS scaling. Fig. 3 depicts the VAS (Fig. 3A) and VRS (Fig. 3B) of patients in each experimental group. As compared with the control group the VAS and VRS pain score was significantly reduced (P<0.01) in the ket+bupi group at 24 and 48 h. Similarly, VAS and VRS pain scores (at 24 and 48 h) were significantly reduced (P<0.05) in the ket and bupi groups compared with the control group.
Figure 3.
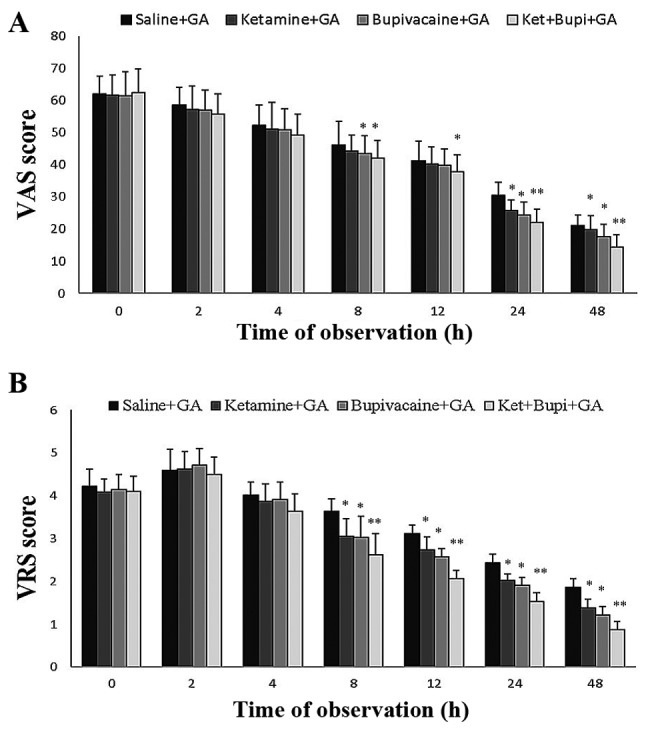
The (A) VAS and (B) VRS of patients in each experimental group. Values were expressed as means ± SD. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 vs. saline+GA group. VAS, visual analog scale; VRS, verbal rating scale; GA, general anesthesia; Ket+Bupi, ketamine and bupivacaine.
Satisfactory score
The satisfactory score of patients is presented in Fig. 4. The ket and bupi groups (at 8, 12, 24 and 48 h) exhibited significantly increased satisfactory scores (P<0.05) as compared with the control group. However, infusion of ket+bupi at 8, 12, 24 and 48 h exhibited a more marked significant increase (P<0.01) vs. control, than the ket or bupi groups.
Figure 4.
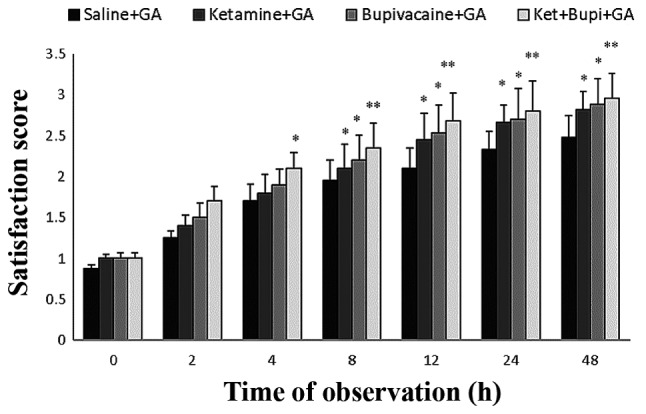
The satisfaction score of patients in each experimental group. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 vs. saline+GA group. GA, general anesthesia; Ket+Bupi, ketamine and bupivacaine.
Ambulation time, knee flexion and hospital stay
Table II presents the ambulation time, knee flexion and hospital stay of patients in the experimental groups. The ambulation time for ket+bupi injected patients (11.10 h), was significantly lower than the control group (18.24 h; P<0.01). Likewise, ket (15.64 h) or bupi (13.84 h) patients exhibited markedly shorter ambulation time than the control group (bupi, P<0.05). However, ket+bupi exhibited the shortest time of ambulation than any other group. In addition, the mean knee flexion degree was significantly greater in ket+bupi group as compared with the control group (P<0.05). Furthermore, the length of hospitalization for ket+bupi group patients was significantly shorter than the control group (P<0.01) and markedly shorter than the ket and bupi groups, suggesting that ket+bupi patients demonstrated faster recovery in terms of physical activity than other groups.
Table II.
The duration (ambulation and hospital stay) and flexion angle of patients in each experimental group.
| Parameters | Saline+GA (n=23) | Ketamine+GA (n=21) | Bupivacaine+GA (n=20) | Ket+bupi+GA (n=20) |
|---|---|---|---|---|
| Time of ambulation, h | 18.24±11.45 | 15.64±9.12 | 13.84±10.03a | 11.10±8.92b |
| Knee flexion, ° | 64.12±09.11 | 67.78±9.34 | 68.11±10.11 | 69.77±9.77a |
| Length of hospital stay, days | 5.50±10.00 | 5.00±11.00 | 4.75±9.00a | 4.20±8.00b |
Data are presented as the mean ± standard deviation.
P<0.05
P<0.01 vs. saline+GA. GA, general anesthesia; ket+bupi, ketamine and bupivacaine.
Adverse events
The incidence of the adverse events in patients are presented in Table III. A number of patients in the control group experienced higher incidence of various adverse effects, including sedation (26.0%; P<0.05), dizziness (17.3%; P<0.05), urinary retention (17.3%; P<0.01), post-operative nausea and vomiting (30.4%; P<0.01) and constipation (13.0%; P<0.05) than other groups. However, the percentage of adverse effects were found to be lesser in ket+bupi group than the other group (only 5 patients experienced the adverse effects). Adverse events in the ket and bupi groups were also reduced as compared with the control group.
Table III.
The incidence of various adverse outcomes data of patients in each experimental group.
| Variables | Saline+GA (n=23) | Ketamine+GA (n=17) | Bupivacaine+GA (n=16) | Ket+bupi+GA (n=16) |
|---|---|---|---|---|
| Sedation | 6 (26.00)a | 3 (17.00) | 3 (18.70) | 1 (6.25) |
| Sweating | 1 (4.30)a | 1 (5.88) | 0 | 0 |
| Constipation | 3 (13.00)a | 0 | 1 (6.25) | 0 |
| Dizziness | 4 (17.30)a | 3 (17.00)c | 2 (12.50) | 1 (6.25) |
| Urinary retention | 4 (17.30)b | 3 (17.00)c | 2 (12.50) | 1 (6.25) |
| PONV | 7 (30.40)b | 4 (23.50)c | 4 (25.00)d | 2 (12.50) |
Data are presented as numbers of patients (%).
P<0.05
P<0.01 vs. saline+GA
P<0.05 vs. ketamine+GA
P<0.05 vs. bupivacaine+GA. GA, general anesthesia; ket+bupi, ketamine and bupivacaine; PONV, post-operative nausea and vomiting.
Discussion
TKA is one of the most effective medical interventions for end-stage chronic knee pain. However, pain control and management following TKA is a crucial factor, which allows faster recovery/ambulation and physiotherapy progress, and enhances patient satisfaction (27). The American Society of Anesthesiologists Task Force on Pain Management currently recommended a multimodal pain regimen (28); thus, the present randomized trial was designed to investigate the possible post-operative pain control effect of a multimodal regimen following TKA. For the present study, normal bupivacaine was used as compared to liposomal bupivacaine, as Surdam et al (23) recently concluded that iposomal bupivacaine is no more effective at reducing pain than normal bupivacaine treatment. In addition, normal bupivacaine is less expensive and is effective as a local anesthetic agent. Similarly, unilateral TKA was preferred for the present trial as it has previously been demonstrated that optimal recovery profiles occur with unilateral positioning, rather than bilateral TKA (5,27). The findings of the present study suggested that IA injection of ket+bupi could ameliorate abolish levels of opioids consumption, pain score (VRS/VAS), time of ambulation, hospital stay and adverse events following infusion of ket+bupi combination, compared with control, or ketamine or bupivacaine alone. In addition, the satisfaction score and knee flexion degree were significantly improved in ket+bupi injected patients, indicating a more expedient post-operative knee rehabilitation process. For the current trial, a single dose of ketamine, bupivacaine or ket+bupi was administered via IA [as IA administration is simple and safe, with fewer systemic side effects and a better mobility rate (23)] based on the positive findings of Ritter et al (29) and Lombardi et al (30), which concluded that IA injection of different analgesic combination can effectively lower pain and improve knee movement following TKA by directly acting on the opioid receptors in the synovial membrane. No significant changes were observed in any of the demographic data among all groups.
Patients administered with ket+bupi exhibited a significant decrease in the levels of total opioid consumption than other experimental patients. Previously, ketamine was reported to exhibit a superior morphine-sparing effect due to its central sensitization property (17). Furthermore, Batra et al (19) previously indicated that ket+bupi would consume fewer opioids due to its enhanced morphine sparing effect. In the present study, patients treated with ket+bupi suffered less pain at 12, 24 and 48 h (following surgery) as compared with other groups (control, ket or bupi). At 48 h the pain score was considerably lowered than 12 or 24 h in all experimental groups. Ketamine decreases post-operative pain up to 48 h, which may be due to its morphine sparing effect as it blocks NMDA receptor and thus reduces the pain intensity (31,32). In addition, Sun et al (18) recently indicated that IA administration of bupivacaine may concomitantly lower the pain intensity in arthroscopic knee surgery. However, in the current study, the combination of ket+bupi yielded a greater analgesic effect than ketamine or bupivacaine alone, which may be due to the adjuvant effect of ketamine (blocking NMDA receptors), which may enhance opiate tolerance and thereby further abolish the pain intensity (19,33).
Ket+bupi at 8, 12, 24 and 48 h exhibited a greater satisfaction score than the control, ket or bupi groups. As detailed above, ket+bupi exhibited the highest opioid sparing effect, which contributes to lesser pain intensity and thus results in the highest satisfactory score. These results are in accordance with the results of Inanoglu et al (21).
The ambulation time and length of the hospital stay for ket+bupi patients are significantly shorter than the control, ket or bupi groups; thus further endorsing the efficient rehabilitation property of ket+bupi combination. The mean knee flexion degree was significantly greater in the ket+bupi group as compared with other experimental groups. The improved pain controlling property of ket+bupi combination is a result of both ketamine and bupivacaine being beneficial following orthopedic surgery (34,35).
Previous studies have demonstrated that the use of opioids may lead to numerous adverse effects including nausea, vomiting, dizziness, urinary retention, sedation and thus an impact on early recovery (10,17). Similarly, in the present study, the majority of control patients exhibited various adverse effects. However, the incidence of adverse effects was found to be reduced in the ket+bupi group, compared with other groups, which may be due to a lesser total opioid consumption rate. Overall, ket+bupi-treated patients exhibited less pain, adverse effects and opioid consumption, with improved post-operative pain management due to its synergic analgesic activity. The outcome of the present study is supported by the findings of Wu et al (36), as they also demonstrated that low doses of ketamine, morphine, and bupivacaine efficiently suppress all nociceptor transduction pathway, and thus prevent central sensitization and improve post-operative pain. The present trial had a number of limitations, as it focused only on immediate follow-up patients in a post-operative/anesthesia care unit. As the present study was a pilot study, acute post-operative pain management was focused on; pre- and post-operative pain comparison has not been performed. The present study also mostly examined acute post-operative pain management, and not chronic pain management as no follow-up was performed. In addition, only a single dose of ketamine, bupivacaine or ket+bupi was administered in the present trial.
In conclusion, the present study demonstrated that the ket+bupi group exhibited better post-operative pain control following TKA through improving pain control and patient satisfaction, and decreasing opioid consumption and adverse effects, thereby facilitating early knee mobilization as compared with other groups, due to synergic analgesic activity. Therefore, the multimodal analgesic regimen (ket+bupi) may be useful in mitigating post-operative pain and improving knee mobilization following TKA. Further studies with a different dosage, perhaps in a larger clinical trial, are required to justify the mechanism behind the synergic analgesic effect of those drugs.
Acknowledgements
The authors would like to thank all the participants involved in the present study.
Funding
The present clinical trial was supported by the Hanzhong Hospital of Traditional Chinese Medicine (Hanzhong, China; grant no. HH-84920).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and HJ desiged the experiments; KS and JZ conducted the experimental trial; KS and HJ collected and analysed the data; and JZ, KS and HJ drafted the manuscript.
Ethics approval and consent to participate
The present study was approved by the Scientific Ethics Committee of Hanzhong Hospital of Traditional Chinese Medicine (Hanzhong, China), and patients provided written informed consent.
Consent for publication
Patients provided written informed consent.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, Rasmussen S. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373:1597–606. doi: 10.1056/NEJMoa1505467. [DOI] [PubMed] [Google Scholar]
- 2.Wang CJ, Long FY, Yang LQ, Shen YJ, Guo F, Huang TF, Gao J. Efficacy of perineural dexamethasone with ropivacaine in adductor canal block for post-operative analgesia in patients undergoing total knee arthroplasty: A randomized controlled trial. Exp Ther Med. 2017;14:3942–3496. doi: 10.3892/etm.2017.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Affas F, Nygårds EB, Stiller CO, Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: A randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthop. 2011;82:441–447. doi: 10.3109/17453674.2011.581264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrington JW, Dalury DF, Emerson RH, Jr, Hawkins RJ, Joshi GP, Stulberg BN. Improving patient outcomes through advanced pain management techniques in total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2013;42(10 Suppl):S1–S20. [PubMed] [Google Scholar]
- 5.Baker PN, Van der Meulen JH, Lewsey J, Gregg PJ. National Joint Registry for England and Wales: The role of pain and function in determining patient satisfaction after total knee replacement. J Bone Joint Surg Br. 2007;89:893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 6.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, Murthy Y. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: A meta-analysis of randomized controlled trials. Anesthesiol. 2010;113:1144–1162. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 7.Lee JK, Chung KS, Choi CH. The effect of a single dose of preemptive pregabalin administered with COX-2 inhibitor: A trial in total knee arthroplasty. J Arthroplasty. 2015;30:38–42. doi: 10.1016/j.arth.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Yu B, Hu X, Zou T, He M, Cai G. Effects of postoperative continuous femoral nerve block analgesia with braun continuous peripheral nerve block catheter set versus novel needle-over-cannula after total knee arthroplasty. Med Sci Monit. 2015;21:1843–1849. doi: 10.12659/MSM.893617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Zhang L, Yang H. Perioperative administration of selective cyclooxygenase-2 inhibitors for postoperative pain management in patients after total knee arthroplasty. J Arthroplasty. 2013;28:207–213.e2. doi: 10.1016/j.arth.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: A prospective randomized controlled trial. J Arthroplasty. 2014;29:329–34. doi: 10.1016/j.arth.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Beaupre LA, Johnston DB, Dieleman S, Tsui B. Impact of a preemptive multimodal analgesia plus femoral nerve blockade protocol on rehabilitation, hospital length of stay, and postoperative analgesia after primary total knee arthroplasty: A controlled clinical pilot study. ScientificWorldJournal. 2012;2012:273821. doi: 10.1100/2012/273821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, et al. Management of Postoperative Pain: A clinical practice guideline from the American pain society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Zeidan A, Kassem R, Nahleh N, Maaliki H, El-khatib M, Struys MM, Baraka A. Intraarticular tramadol-bupivacaine combination prolongs the duration of postoperative analgesia after outpatient arthroscopic knee surgery. Anesth Analg. 2008;107:292–299. doi: 10.1213/ane.0b013e31816ba364. [DOI] [PubMed] [Google Scholar]
- 14.Bondok RS, El-Hady Abd AM. Intra-articular magnesium is effective for postoperative analgesia in arthroscopic knee surgery. Br J Anaesth. 2006;97:389–392. doi: 10.1093/bja/ael176. [DOI] [PubMed] [Google Scholar]
- 15.Jouguelet-Lacoste J, La Colla L, Schilling D, Chelly JE. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: A review of the current literature. Pain Med. 2015;16:383–403. doi: 10.1111/pme.12619. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Lin H, Yi WB. Evaluation of the effects of ketamine on spinal anesthesia with levobupivacaine or ropivacaine. Exp Ther Med. 2016;12:2290–2296. doi: 10.3892/etm.2016.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam F, Chauvin M, Manoir Du B, Langlois M, Sessler DI, Fletcher D. Small dose ketamine improves postoperative analgesia and rehabilitation after total knee arthroplasty. Anesth Analg. 2005;100:475–480. doi: 10.1213/01.ANE.0000142117.82241.DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun QB, Liu SD, Meng QJ, Qu HZ, Zhang Z. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2015;16:21. doi: 10.1186/s12891-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batra YK, Mahajan R, Bangalia SK, Nagi ON, Dhillon MS. Bupivacaine/ketamine is superior to intra-articular ketamine analgesia following arthroscopic knee surgery. Can J Anesth. 2005;52:832–836. doi: 10.1007/BF03021778. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Szulc A, Walton S, Bosco J, Iorio R. Pain control and functional milestones in total knee arthroplasty: Liposomal bupivacaine versus femoral nerve block. Clin Orthop Relat Res. 2017;475:110–117. doi: 10.1007/s11999-016-4740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inanoglu K, Akkurt Ozbakis BC, Turhanoglu S, Okuyucu S, Akoglu E. Intravenous ketamine and local bupivacaine infiltration are effective as part of a multimodal regime for reducing post-tonsillectomy pain. Med Sci Monit. 2009;15:CR539–CR543. [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30:325–329. doi: 10.1016/j.arth.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Li L, Xu YT, Wang X. Intravenous analgesics for pain management in postoperative patients: a comparative study of their efficacy and adverse effects. Trop J Pharma Res. 2016;15:1799–806. doi: 10.4314/tjpr.v15i8.29. [DOI] [Google Scholar]
- 26.Matassi F, Duerinckx J, Vandenneucker H, Bellemans J. Range of motion after total knee arthroplasty: The effect of a preoperative home exercise program. Knee Surg Sports Traumatol Arthrosc. 2014;22:703–709. doi: 10.1007/s00167-012-2349-z. [DOI] [PubMed] [Google Scholar]
- 27.Tali M, Maaroos J. Lower limbs function and pain relationships after unilateral total knee arthroplasty. Int J Rehab Res. 2010;33:264–267. doi: 10.1097/MRR.0b013e3283352126. [DOI] [PubMed] [Google Scholar]
- 28.American Society of Anesthesiologists Task Force on Acute Pain Management: Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 29.Ritter MA, Koehler M, Keating EM, Faris PM, Meding JB. Intra-articular morphine and/or bupivacaine after total knee replacement. J Bone Joint Surg Br. 1999;81:301–303. doi: 10.1302/0301-620X.81B2.0810301. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–30. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]
- 31.Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anesth. 2011;58:911–923. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- 32.Elia N, Tramer MR. Ketamine and postoperative pain-a quantitative systematic review of randomized trials. Pain. 2005;113:61–70. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 33.Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology: J Am Soc Anesthesiol. 2005;102:211–20. doi: 10.1097/00000542-200501000-00030. [DOI] [PubMed] [Google Scholar]
- 34.Menigaux C, Guignard B, Fletcher D, Sessler DI, Dupont X, Chauvin M. Intraoperative small-dose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–612. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Isharnouby NM, Eid HE, Elezz Abou NF, Moharram AN. Intraarticular injection of magnesium sulphate and/or bupivacaine for postoperative analgesia after arthroscopic knee surgery. Anesth Analg. 2008;106:1548–1552. doi: 10.1213/ane.0b013e31816a67a8. [DOI] [PubMed] [Google Scholar]
- 36.Wu CT, Yeh CC, Yu JC, Lee MM, Tao PL, Ho ST, Wong CS. Pre-incisional epidural ketamine, morphine and bupivacaine combined with epidural and general anaesthesia provides pre-emptive analgesia for upper abdominal surgery. Acta Anaesthesiol Scand. 2000;44:63–68. doi: 10.1034/j.1399-6576.2000.440112.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


