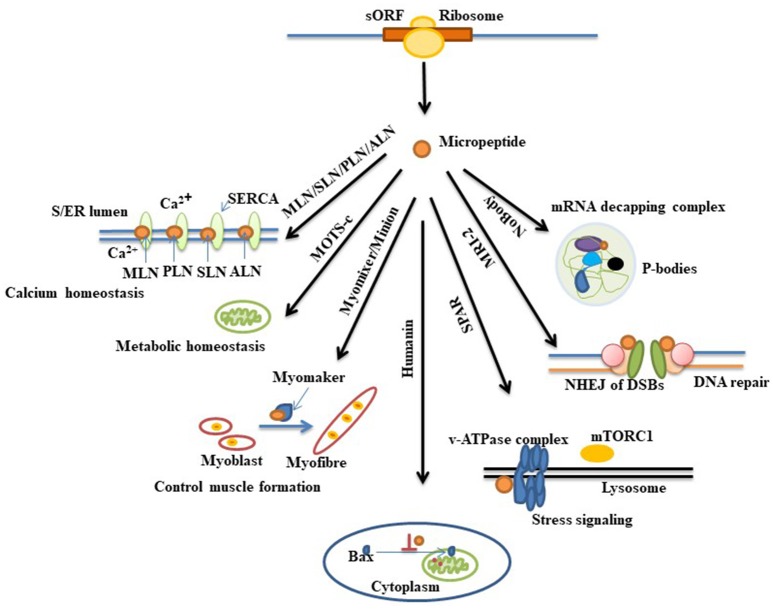
Figure 1.
Diverse biological function of recently annotated micropeptides. Micropeptides are found to be involved in many biological processes. Myoregulin (MLN), phospholamban (PLN), sarcolipin (SLN), and another regulin (ALN) are a group of peptides that interact with the protein SERCA (a Ca2+ Pump) in sarcoplasmic and endoplasmic reticulum (S/ER) and maintain Ca2+ homeostasis in the cell. MOTS-c and humanin are mitochondrial sORF-encoded micropeptides that display important roles in metabolic homeostasis and apoptosis, respectively. Humanin suppresses apoptosis by preventing the translocation of an apoptosis inducing protein, Bax (Bcl2-associated X protein), from cytoplasm to mitochondria. Another micropeptide named MRI-2 is found to enhance non-homologous end joining (NHEJ) of double-strand DNA breaks (DSBs) by associating with other DNA end-binding proteins (Ku proteins). Myomixer, minion, SPAR, and NoBody, four other micropeptides that have been recently discovered, have distinct biological roles wherein myomixer and minion stimulate the fusion of myoblast to form myofiber during muscle formation by participating with another protein, myomaker. The micropeptide SPAR is localized into lysome where it interacts with the lysosomal v-ATPase complex and regulates mTORC1 protein activation during stress signaling. NoBody, a p-body (processing-body, which is involved in mRNA turnover) dissociating micropeptide, shows its function by interacting with the mRNA decapping complex.