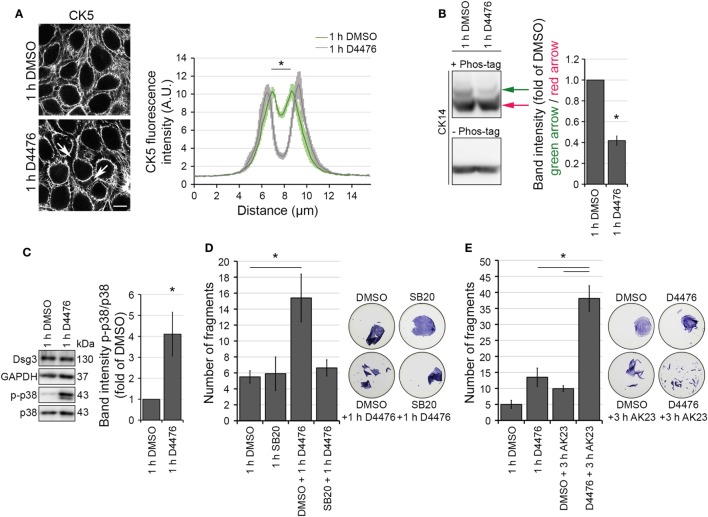
Figure 4.
Casein kinase 1 inhibition provoked keratin alterations resembling the PV-IgG-induced phenotype. HaCaT-CK5 cells were incubated with CK-1 inhibitor D4476 for 1 h. The resulting keratin retraction (arrows) was measured with a bar of 15 µm length perpendicular to the cell border of two adjacent cells (A) (n = 75–100 cells from three to four independent experiments, *p < 0.05 vs. 1 h DMSO). Bar is 10 µm. (B) Phos-tag™ was incorporated in a 6% Western blot gel (+ Phos-tag) to detect the phosophorylation status of CK14 in HaCaT-CK5 cells. Control gel without Phos-tag (– Phos-tag) excluded protein fragmentation. Band intensity of both phosphorylation sites was measured and the first phosphorylation site (green arrow) was divided by the basal phosphorylation site (red arrow) (n = 4, *p < 0.05 vs. 1 h DMSO). (C) SDS lysates were generated from cells treated with 1 h D4476 or 1 h DMSO and p38MAPK phosphorylation was evaluated. Densitometric results were normalized to total p38 levels as a loading control (n = 6, *p < 0.05 vs. 1 h DMSO) Cell adhesion was quantified in HaCaT keratinocytes by dispase-based dissociation assays. Pre-incubation with SB203580 (SB20) as a specific inhibitor of p38MAPK or DMSO as a control for 1 h was followed by D4476 incubation for 1 h (n = 4–5, *p < 0.05) (D). Pre-incubation with D4476 or DMSO for 1 h followed by 3 h incubation with AK23, a pathogenic monoclonal desmoglein (Dsg)3 antibody (E) (n = 4, *p < 0.05). Remaining cell sheets and fragments were stained with 10 µM MTT for visualization.