Figure 1.
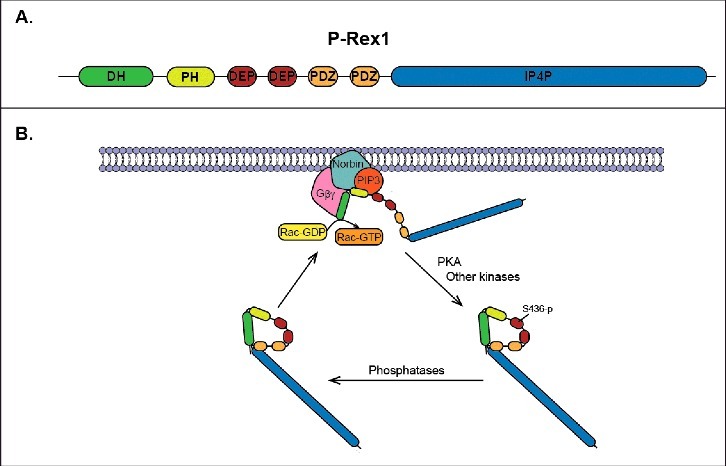
Regulation of P-Rex1 activity. (A) Domain structure of P-Rex1 showing the DH-PH tandem, PDZ domains, DEP domains, and IP4P-like domain. (B) Proposed cycle of activation/deactivation of P-Rex1. In response to receptor activation, inactive P-Rex1 in the cytoplasm is recruited to the plasma membrane by direct interactions with PIP3, Gβγ subunits and norbin. The interactions with norbin and PIP3 occur via the PH domain, while Gβγ subunits bind directly to the DH domain. At the plasma membrane P-Rex1 GEF-activity toward Rac is stimulated by PIP3, Gβγ and norbin, thus resulting in the release of GDP from Rac and the binding of GTP. PKA phosphorylates P-Rex1 at the plasma membrane in Ser436 located in the first DEP domain, which results in intramolecular autoinhibitory interactions between the DH-PH tandem and the first DEP domain.
