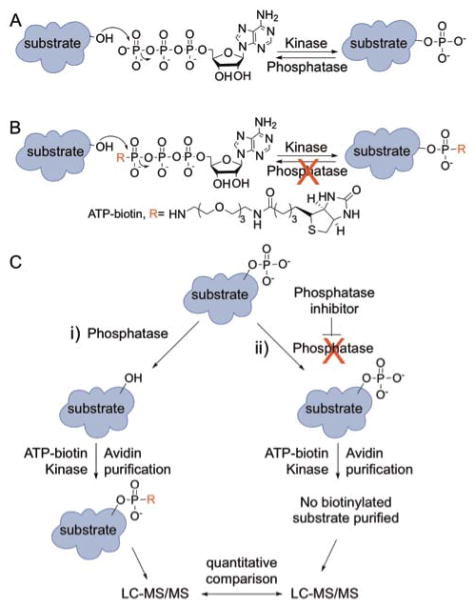
Figure 1.
Protein phosphorylation and the K-BIPS method. (A) Kinases transfer a phosphoryl group from ATP to cellular proteins. Phosphatases reverse the reaction. (B) Kinases utilize γ-modified ATP analogs, such as ATP-biotin, to covalently modify substrate proteins. The γ-modified phosphoryl group once attached is resistant to phosphatase degradation. (C) The K-BIPS method. Phosphatases present in lysates remove phosphoryl groups from already phosphorylated substrates (i, left path). However, when a phosphatase is inactivated by inhibitor treatment, the substrates are maintained in the phosphorylated form (ii, right path). (i) Phosphatase substrates in the untreated lysates are biotinylated by ATP-biotin and active kinases. After avidin purification of biotinylation proteins, LC-MS/MS analysis will identify the captured substrates. (ii) Phosphorylated substrates in the phosphatase-inhibited sample are immune to biotinylation by ATP-biotin. After avidin purification and LC-MS/MS analysis, quantitative comparison of proteins present at elevated levels in the untreated sample versus the phosphatase inhibitor-treated sample will reveal possible substrates.