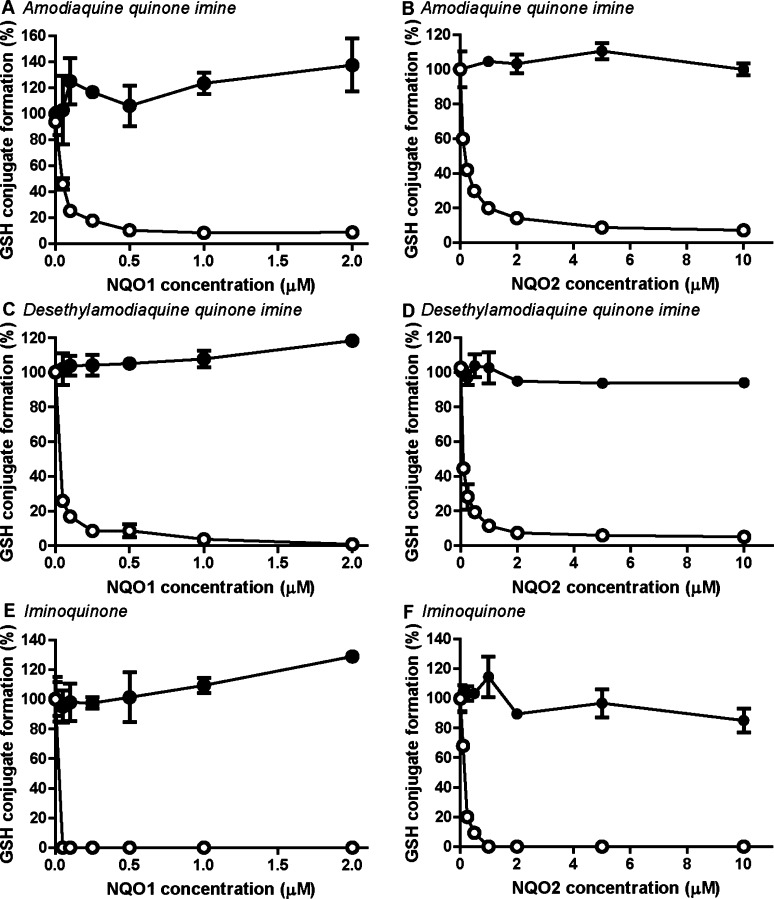
Figure 5.
NQO2-catalyzed reduction of amodiaquine- and carbamazepine-derived quinones. Recombinant NQO1 or NQO2 was incubated for 1 min with purified quinone imines (50 μM) and GSH (50 μM) in absence (filled circles) or presence (open circles) of 250 μM NADPH or NRH. Amodiaquione quinone imine (A and B) and desethylamodiaquine quinoneimine (C and D) are formed by CYP-catalyzed oxidation of amodiaquine, and iminoquinone is a metabolite of carbamazepine (E and F). GSH conjugation is presented relative to the incubation without NQO and cofactor. Data represent the average and range of duplicates.