Figure 3.
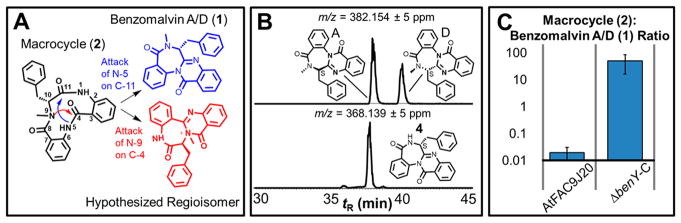
Transannulation is mediated by BenY-CT. (A) Benzodiazepine formation could occur through two different transannulation paths. The path leading to benzomalvin A/D (1) is colored blue. The path leading to an undetected and unreported alternate regioisomer with a quaternary amide is colored red. (B) The top trace shows two chromatographic peaks in the extracted ion chromatogram for m/z 382.1547, corresponding to the atropisomeric pair of benzomalvin A and benzomalvin D, which are in conformational equilibrium and are collectively called benzomalvin A/D (1). The bottom trace shows the extracted ion chromatogram for desmethyl benzomalvin (4), which shows only one major chromatographic peak. Benzomalvin regioisomerism is expected to lead to four peaks and two peaks of similar abundance in the top and bottom traces, respectively, suggesting transannulation occurs with regioselectivity, regardless of methylation status. (C) The compound 2:compound 1 ratio is >100-fold increased by deletion of benY-CT, suggesting BenY-CT catalyzes transannulation of 2 to form 1. The p value for the difference between AtFAC9J20 and ΔbenY-C was calculated by a Student’s t test and equals 0.004, indicating a statistically significant difference.