Abstract
Morphogenesis is a complex and highly coordinated process orchestrated by temporal spatial activity of developmental pathways. How the different pathways interact to guide the developmental program remains an intriguing and open question. MAP3K1-JNK and Wnt are signaling pathways crucial for embryonic eyelid closure, an epithelial morphogenetic event conserved in mammals. Here we used a mouse model of eyelid development and genetic and biochemistry tools to investigate the relationships between the two pathways. We found that Wnt activation repressed MAP3K1 expression. Using Axin-LacZ reporter mice, spatial Wnt activity was detected in the leading edge of the developing eyelid. Conditional knockout of Wntless (Wls) in ocular surface ectoderm blocked eyelid formation, and significantly increased MAP3K1 expression in eyelid cells at the nasal canthus region. Conversely, knockout of Dkk2, encoding a canonical Wnt antagonist, resulted in an increase of Wnt activity in cells at the upper eyelid margin near the nasal canthus. Up-regulation of Wnt signaling in the Dkk2-knockout embryos corresponded to down-regulation of MAP3K1 expression. In vitro data showed that Wnt3a treatment decreased MAP3K1 promoter activity, whereas activation of Wnt by lithium chloride inhibited MAP3K1 expression, and attenuated MAP3K1-mediated JNK activity. Our data identify a unique signal crosstalk between Wnt signaling and the MAP3K1-JNK pathway in epithelial morphogenesis.
Keywords: Embryonic eyelid closure, MAP3K1, canonical Wnt signaling, Wntless, DKK2, JNK
Graphical Abstract
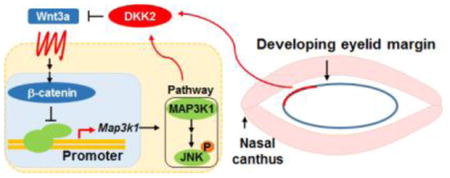
Introduction
Morphogenesis is a fundamental event in the development of multicellular organisms. It is dictated by genetic and environmental factors, and regulated by specific signal transduction pathways. A diverse array of signaling pathways are tightly regulated and spatiotemporally activated during development. These pathways interact extensively to guide the complexity, plasticity and order of morphogenesis (Cvekl and Zhang, 2017). Coordinated pathway activities are crucial for the developmental processes that give rise to tissues and/or organs. While the combinatorial action of the pathways controls cell behaviors, such as proliferation, differentiation, shape change and migration, the crosstalk relationship between individual pathways is multifaceted and poorly understood. Understanding the morphogenetic control, and deciphering its underlying signaling machinery has far reaching clinical implications in the health of embryonic life. Moreover, such information may pave the way for improving therapies in adult diseases, such as tissue injury and malignancy, associated with aberrant activities of the developmental pathways.
Embryonic eyelid closure represents an advantageous system for studying the regulatory mechanisms underlying morphogenesis. In mice, eyelid closure occurs between embryonic day E15 – E16, when the opposing eyelids move forward and ultimately meet and fuse, forming a closed eyelid that covers the ocular surface (Barishak, 1992; Harris and Juriloff, 1986; Tao et al., 2006). This process is driven primarily by morphological change and migration of the epithelial cells located at the developing eyelid margin (Behrens et al., 1999; De Moerlooze et al., 2000; Heller et al., 2014; Threadgill et al., 1995; Xia and Karin, 2004). The mouse eyelid remains closed at birth. At postnatal day (P) 12–14, the lid fusion junction breaks up, separating the upper and lower eyelids. Because eyelid closure is a late morphogenetic event in mammalian embryogenesis, its defect is not life threatening but results in an eye open at birth (EOB) phenotype. The phenotype is extremely easy to spot as soon as the pups are born. Consequently, the EOB defect has been detected in more than 150 genetic mutant strains (http://www.informatics.jax.org/mp/annotations/MP:0001302). Over the years, mice with the EOB phenotype have been used as tools to delineate the genetic architecture and molecular mechanisms underlying eyelid development and epithelial morphogenesis (Rubinstein et al., 2016).
Genetic data have provided compelling evidence that eyelid morphogenetic closure is regulated by an extremely complex signaling network (Huang et al., 2009; Xia and Karin, 2004). At least eight major signaling pathways, including fibroblast growth factor (FGF), epidermal growth factor (EGF), bone morphogenetic protein (BMP), glucocorticoid receptor (GR), G-protein-coupled receptor (GPCR), Wnt/β-catenin, RhoA/ROCK, and MAP3K1/JNK, are implicated in eyelid closure (Berkowitz et al., 1996; Gage et al., 2008; Huang et al., 2009; Kumar and Duester, 2010; Lee et al., 2003; Mine et al., 2005; Nakagawa et al., 1996; Vassalli et al., 1994). Some pathways appear to be interconnected, while others display a high degree of compartmentalization and spatial segregation (Geh et al., 2011; Huang et al., 2009; Takatori et al., 2008; Wu et al., 2012; Xia and Karin, 2004). The precise relationships of the pathways and exactly how the different pathways interact in vivo are still poorly understood.
MAP3K1, also known as MEKK1, is a member of the MAP3K superfamily, responsible for signal transduction leading to the activation of specific MAPKs (Uhlik et al., 2004). MAP3K1 is highly expressed in the epithelial cells of the embryonic eyelid margin where it is required for activation of the JNK MAPKs, and induction of cell shape change and actin cytoskeleton reorganization (Zhang et al., 2003). Genetic inactivation of MAP3K1 in mice leads to the EOB phenotype (Yujiri et al., 2000; Zhang et al., 2003). Detailed molecular analyses have revealed that MAP3K1 acts as a nexus integrating multiple developmental signals, such as Activin B and EGF, and connecting the RhoA and JNK signaling pathways (Geh et al., 2011; Meng et al., 2014b; Takatori et al., 2008; Zhang et al., 2003). Recently, MAP3K1 has been shown to also integrate signals derived from environmental toxic agents (Mongan et al., 2015). Dioxin-like environmental pollutants synergize with Map3k1 allelic mutation to inhibit the JNK pathway and block eyelid closure. Dioxin toxicity is mediated by the aryl hydrocarbon receptor (AHR), a ubiquitous cellular receptor for various toxicants, but how the dioxin-AHR axis links to the MAP3K1-JNK pathway at the molecular level is yet to be understood. By integrating diverse developmental and environmental signals, MAP3K1 acts as a key player in the molecular circuitry governing embryonic eyelid closure.
The canonical Wnt/β-catenin pathway is a central morphogenetic mechanism regulating vertebrate development. It plays an essential role in the control of cell proliferation and cell fate determination in multiple developmental processes (Carpenter et al., 2015). Wnt signaling has a profound effect on the development of all eye tissues that includes the morphogenesis of the eyelids (Liu et al., 2003). Various Wnt ligands are expressed in the developing eyelids and Wnt signaling is required for eyelid development in multiple stages, including lid formation, growth and closure (Gage et al., 2008; Guo et al., 2018; Liu et al., 2003; Wang et al., 2006; Wu et al., 2012). Dysregulation of Wnt/β-catenin signaling results in defective embryonic eyelid closure. For example, knockout of Dkk2, encoding a Wnt antagonist, in mice leads to the EOB phenotype, suggesting that inhibition of the Wnt pathway by DKK2 is required for eyelid closure (Gage et al., 2008; Meng et al., 2014a). Conversely, however, mice carrying Tcf3 loss of function mutation, which results in inhibition of Wnt/β-catenin signaling, also exhibit a partial EOB phenotype (Wu et al., 2012). Notably, in E15.5 Tcf3 mutant embryos, Wnt inhibition occurs only in a small group of eyelid epithelial cells located near the mucocutaneous junction, suggesting a high degree of cell type specificity of TCF3-mediated Wnt activity. Hence, the canonical Wnt pathway appears to be regulated by distinct mechanisms in a spatial–temporal specific manner during eyelid development.
Our previous in vitro data suggest that MAP3K1 represses Wnt signaling, because loss of MAP3K1’s kinase domain leads to increased induction of β-catenin activity (Jin et al., 2013). Here we looked further into the relationship between these pathways by focusing mainly on the embryonic eyelids and using a combination of genetic, molecular, histological and imaging analyses. We identified a unique link between MAP3K1 and Wnt. Specifically, we showed that activation of the Wnt pathway leads to repression of Map3k1 promoter and gene expression, and reduction of MAP3K1-dependent activation of the JNK MAPKs.
Materials and methods
Mouse lines, reagents and antibodies
Map3k1ΔKD/+, Wntless flox mice (WlsF), Dkk2−/− and Le-cre mice were described before (Ashery-Padan et al., 2000; Carpenter et al., 2010; Li et al., 2005; Zhang et al., 2003). The Wnt pathway reporter mouse line Axin2-LacZ (B6N.129P2-Axin2tm1Wbm/J, stock number 009120) was from Jackson Lab. Gestational age was determined based on the detection of a vaginal plug as embryonic day (E) 0.5.
The MAP3K1 antibody was as described (Xia et al., 2000); antibodies for phosphorylated- and total-JNK were from Cell Signaling (Danvers, MA). The β-actin antibody, retinoic acid (RA), phorbol 12-myristate 13-acetate (PMA) and lysophosphatidic acid (LPA) were from Sigma-Aldrich (St. Louis, MO). Wnt5a was from R&D Systems (Minneapolis, MN), transforming growth factor α (TGFα), fibroblast growth factor b (FGFb), epidermal growth factor (EGF), activin B (ActB), transforming growth factor β (TGFβ) and insulin-like growth factor (IGF) were from Peprotech (Rocky Hill, NJ), and sphingosine 1-phosphate (S1P) was from Avanti Polar Lipids (Alabaster, AL).
X-Gal Staining and quantification
Embryos were fixed with 2% paraformaldehlyde (PFA) and 0.2% glutareldahyde phosphate buffer (pH 7.3) supplemented with 5 mM EGTA, 2 mM MgCl2 for 20 minutes. X-gal staining was performed following procedures described before (Zhang et al., 2003). Briefly, embryos were immersed in staining solution containing 1 mg/ml X-gal in 0.1M phosphate buffer (pH 7.3) supplemented with 2 mM MgCl2, 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide. For Axin2-LacZ embryos, staining was carried out at room temperature for 1 hour, and for Map3k1ΔKD/+ embryos staining was carried out at 37°C overnight. Following staining, the embryos were fixed with 4% PFA at 4°C overnight. Images were captured using a Leica MZ16FA microscope and pixel density values in images were measured using Axiovision software.
Cell culture, β-galactosidase assay, luciferase assay and Western blotting
The wild type, Map3k1ΔKD/+ and Map3k1ΔKD/ΔKD fibroblasts and embryonic stem cells (ESCs) derived from genetic mutant mouse embryos were described before (Geh et al., 2011; Xia et al., 2000). The human embryonic kidney 293 (HEK293) and keratinocyte HaCaT cell lines were originally from the American Type Culture Collection (ATCC). The Wnt3a expression plasmid or empty vector (pCDNA3.1) and the reporter plasmids, including Map3k1 promoter drive luciferase construct (pMap3k1-luc), Cytomegalovirus (CMV) -renilla luciferase reporter, and wild-type and mutant TCF/LEF DNA-binding sites-driven luciferase reporter (TCF/LEF-luc and TCF/LEF-mut-luc), were described elsewhere (Geh et al., 2011; Jin et al., 2013).
Cells were seeded in 24-well tissue culture plates at a density of 1×105 cells/well and grew for 24 hours. The cells were transfected with reporter plasmids, including the Firefly-luc and CMV-renilla luciferase reporters and the Map3k1-Luc reporter, and in some experiments, co-transfected with Wnt3a or empty vector plasmids. Transfection was performed using Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Cells were harvested at 48 h after transfection, and in some experiments, they were treated with LiCl for 24 hours before harvesting.
Luciferase activities were determined using the dual-luciferase assay kit (Promega, Madison, WI), following the instructions of the manufacture. The β-galactosidase assay, used to evaluate endogenous MAP3K1-β-GAL fusion protein expression, was performed using the Beta-Glo Assay System (Promega, Madison, WI). Western blotting analysis was used to examine protein expression in cell lysates following a standard protocol as described before (Geh et al., 2011).
Statistical analysis
All data are shown in mean SD of at least three samples. Comparisons between groups were performed using Student’s two-tailed t test. Values of *P<0.05, **P<0.01, and ***P<0.001 were considered statistically significant.
Results
Lithium chloride (LiCl) inhibits MAP3K1 expression
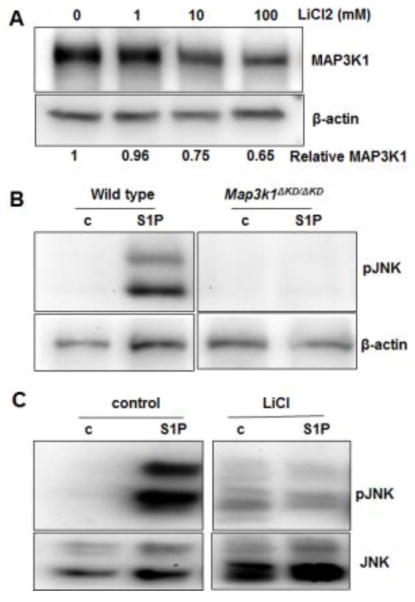
Our previous data have shown that MAP3K1 displays a temporal-spatially distinct pattern of expression during embryonic development (Zhang et al., 2003). To identify the developmental signals responsible for MAP3K1 expression, we used fibroblasts isolated from mice, carrying the Map3k1ΔKD allele, which encodes a truncated kinase-dead MAP3K1-β-gal fusion protein. We measured β-gal activities in Map3k1ΔKD/+ and Map3k1ΔKD/ΔKD fibroblasts treated with stimuli known to activate key developmental signaling pathways. Compared to control, treatment with Wnt5a, retinoic acid (RA), sphingosine 1-phosphate (S1P), phorbol 12-myristate 13-acetate (PMA), transforming growth factor α (TGFα), EGF, FGF, activin B (ActB) and insulin-like growth factor (IGF) did not change β-gal expression, whereas treatment with lysophosphatidic acid (LPA) and transforming growth factor β (TGFβ) caused a slight increase in β-gal activity. Conversely, treatment of cells with Lithium Chloride (LiCl), a potent activator of the canonical Wnt pathway, caused a marked decrease of β-gal activity (Fig. 1A).
Figure 1. Down-regulation of MAP3K1 expression by lithium chloride (LiCl).
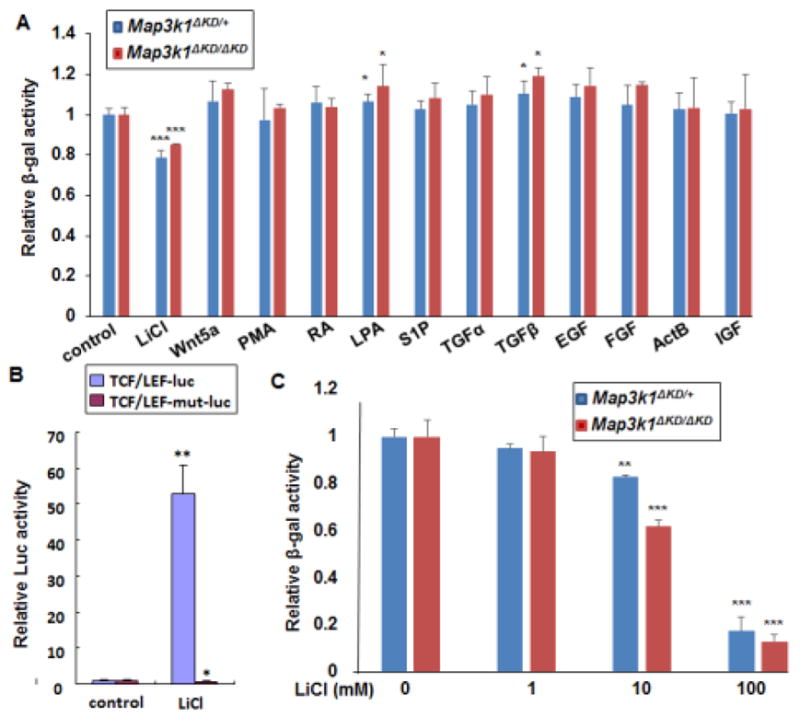
(A and C) Mouse embryonic fibroblasts (MEFs) isolated from mice carrying the knock-in allele, encoding a kinase-dead truncated MAP3K1 protein fused to β-gal (Map3k1ΔKD), as indicated in the figures, were used. The cells were treated for 24 h with (A) different stimuli, including LiCl (10 mM), Wnt5a (100ng/ml), PMA (40nM), RA (10uM), LPA (20uM), S1P (20uM), TGFα (100 ng/mL), TGFβ (10ng/ml), EGF (10 ng/ml), FGF (10 ng/ml), ActB (10 ng/ml) and IGF (100 ng/ml), or (C) various concentrations of LiCl as indicated. The β-gal activities were examined, and relative activities were calculated based on protein concentration. (B) HEK293 cells were co-transfected with CMV-renilla luciferase reporter and Wnt/β-catenin signaling luciferase reporter in its wild type (TCF/LEF-luc) or mutant (TCF/LEF-mut-luc) forms. The cells were treated with LiCl for 24 hours and luciferase activity was examined. The ratio of firefly-luc versus renilla-luc was calculated, and the value in control was set as 1. Statistical significance was determined using Student t-test, * P<0.05, **P<0.01 and ***P<0.001 are considered significantly different from the value in control samples.
LiCl activates Wnt signaling through inhibition of glycogen synthetase kinase-3β (GSK3β) and consequently stabilizing and activating β-catenin (Carmichael et al., 2002). To confirm the role of LiCl in β-catenin activation, we examined the TCF/LEF DNA-binding site-driven luciferase reporters (TCF/LEF-luc). LiCl treatment strongly induced the luciferase activities in HEK293 cells transfected with the TCF/LEF-luc reporters, but was unable to activate a reporter driven by a mutant β-catenin binding site (TCF/LEF-mut-luc) (Fig. 1B).
Because LiCl acts dose-dependently to activate Wnt signaling (Carmichael et al., 2002), we tested different concentrations of LiCl on β-gal activity in the Map3k1ΔKD/+ cells. A significant reduction of β-gal activities was detected in cells treated with 10 mM LiCl, and the reduction was more evident in cells treated with 100 mM LiCl for 24 h (Fig. 1C). Compared to that in the control cells, the β-gal activities in cells treated with 100 mM LiCl were reduced by 80%. Similarly, LiCl dose-dependently decreased β-gal activity in the Map3k1ΔKD/ΔKD fibroblasts. These observations unveil a possible relationship between activation of Wnt signaling and repression of MAP3K1 expression.
Wnt activity and function in the developing eyelid
A well-characterized developmental role of MAP3K1 is the regulation of embryonic eyelid closure, where MAP3K1 is spatially expressed in the developing eyelid (Zhang et al., 2003). To evaluate whether Wnt could have a role in the same developmental process, we first examined Wnt activity using the reporter mice (Axin2-LacZ), in which the β-gal expression is driven by the Wnt-activated Axin2 promoter. In the E14.5 embryos, high level of β-gal activity was detected in multiple tissues, including limbs, ear buds, noses and whisker follicles (Fig. 2A). In addition, strong β-gal activity was evident in the developing eyelids and was particularly abundant in the edge of eyelid opening (Fig. 2B).
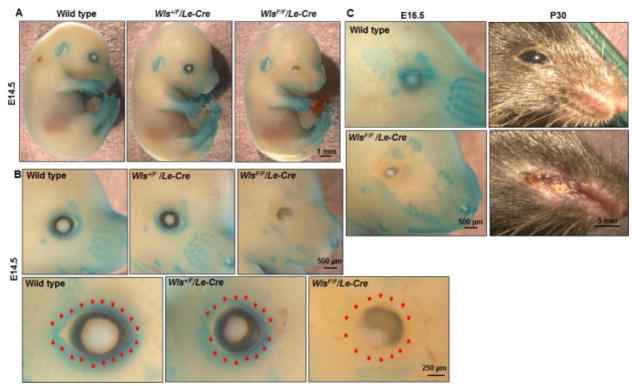
Figure 2. Wnt activity in the developing eyelid.
X-gal staining of the Axin2-LacZ embryos on different genetic backgrounds, including wild type, Wls+/F/Le-Cre, and WlsF/F/Le-Cre. X-gal staining of the E14.5 embryos were photographed at (A) low, or (B) intermediate (upper panels) and high (lower panels) magnifications, and (C) staining of the E16.5 fetuses and P30 eyes were photographed. In the Axin2-LacZ embryos, β-gal expression was detected in the surface of the eye, ear, limbs, and vibrissae. The expression was lost in areas around the eye of the WlsF/F/Le-Cre embryos, which also displayed severely disruption of the overall eyelid structures. Red arrows indicate the eyelid leading edge.
We next crossed Wlsflox (WlsF) and Le-Cre mice and generated pups with Wntless conditionally ablated in the ocular surface ectoderm (Carpenter et al., 2015). Using the Axin2-LacZ as a readout of Wnt activity we showed that the WlsF/F/Le-Cre embryos (WlsF/F/Le-Cre/Axin2-LacZ) had Wnt activity abolished specifically in the developing eyelids (Fig. 2). Correspondingly, the WlsF/F/Le-Cre eyelids were either missing or structurally malformed with the symptoms more severe when the pups grew older (Fig. 2C). In the adult age, the WlsF/F/Le-Cre mice displayed additional eye abnormalities, such as complete destruction of lens and cornea. These data are consistent with previous reports showing that Wnt is activated in the developing eyelids (Liu et al., 2003), and provide additional evidence that lacking Wnt has detrimental effect on eyelid development.
Loss of Wnt up-regulates MAP3K1 expression in vivo
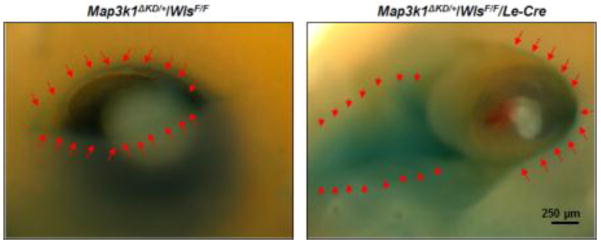
We used the Wntless conditional knockout model to further investigate whether Wnt signaling affected MAP3K1 expression. To this end, we generated Map3k1ΔKD/+/WlsF/F/Le-Cre compound mutants and performed X-gal staining of the embryos to trace MAP3K1 expression. In the presence of Wntless (Map3k1ΔKD/+/WlsF/F), the β-gal positive cells were detected restricted to the leading edge of the developing eyelids, similar to that reported before (Zhang et al., 2003). In the absence of Wntless (Map3k1ΔKD/+/WlsF/F/Le-Cre), the β-gal positive cells were still detectable in the eyelid edge, but were much more abundant in a large area in the nasal canthus region (Fig. 3). These observations provide the first clue that inhibition of Wnt up-regulates MAP3K1 expression in the developing eyelids.
Figure 3. Wnt signaling regulates MAP3K1 expression in vivo.
X-gal staining of the Map3k1ΔKD/+/WlsF/F and Map3k1ΔKD/+/WlsF/F/Le-Cre embryos at E14.5. In the Map3k1ΔKD/+/WlsF/F embryos, the β-gal positive cells were detected restricted to the leading edge of the developing eyelids (left panel, red arrows). In the absence of Wntless (Map3k1ΔKD/+/WlsF/F/Le-Cre), the eye structure was disrupted, and the β-gal positive cells were detected in a larger area, most abundant in the eyelid margin (right panel, red arrows) and nasal canthus region (right panel, red arrowheads).
Activation of Wnt down-regulates MAP3K1 expression in the embryonic eyelids
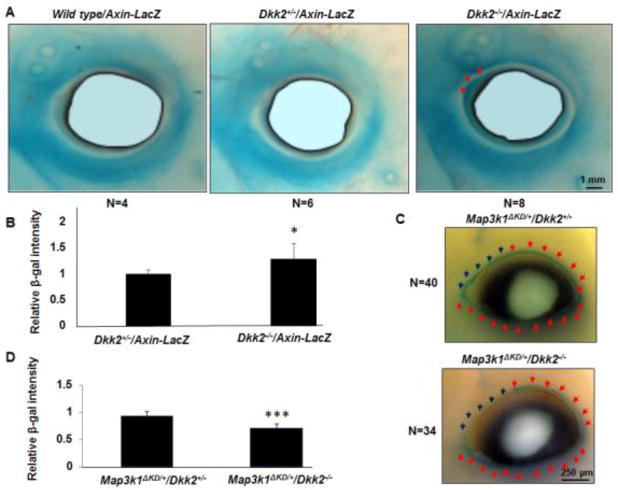
Given the above observations, we speculated that conversely activation of the Wnt pathway might down-regulate MAP3K1 level. To test this possibility, we used mice deficient in DKK2, a secreted Wnt inhibitor that plays an essential role in eyelid development (Gage et al., 2008). Using the Axin2-LacZ reporter mice, we detected β-gal positive cells in the eyelids of wild type, Dkk2+/− and Dkk2−/− embryos (Fig. 4A). While the β-gal activity level was not overtly different between the Dkk2 genotypes, subtle differences were found in specific regions of the eyelids. Compared to the wild type and Dkk2+/− littermates, the Dkk2−/− embryos displayed stronger β-gal activity in the upper eyelids near the nasal canthus (Fig. 4A). Quantification confirmed that the β-gal activity was significantly higher in this region of the Dkk2−/− embryos (Fig. 4B).
Figure 4. Activation of Wnt down-regulates MAP3K1 expression in vivo.
(A) X-gal staining of the Axin2-LacZ E14.5 embryos on different genetic backgrounds, including wild type, Dkk2+/− and Dkk2−/−. The β-gal positive cells were detected in the eyelids of wild type, Dkk2+/− and Dkk2−/− embryos, but with stronger X-gal staining in the upper eyelids next to the nasal canthus of the Dkk2−/− embryos (red arrows). (B) The β-gal intensity in the nasal canthus region was quantified, and was compared to the value in wild type and Dkk2+/− groups set as 1. (C) X-gal staining of the Map3k1ΔKD/+/Dkk2+/− and Map3k1ΔKD/+/Dkk2−/− E14.5 embryos. The β-gal positive cells were detected in the leading edge of the developing eyelids of both groups (blue and red arrows), while Dkk2−/− embryos displayed decreased X-gal staining in the upper eyelids next to the nasal canthus (lower panel, blue arrows). (D) The X-gal staining ratio between blue arrows region versus red arrows region was calculated, and the value of control group (Map3k1ΔKD/+/wild type and Map3k1ΔKD/+/Dkk2+/−) was set as 1. The statistical significance was determined using Student t-test, *P<0.05 and ***P<0.001 are considered significantly different from control, whereas there was no significant differences in values among the control samples.
To evaluate the effect of Wnt activation on MAP3K1 expression, we crossed Dkk2-mutant mice with Map3k1ΔKD mice and examined MAP3K1 expression in the developing eyelid of Map3k1ΔKD/+ embryos using whole mount X-gal staining. Compared to their Dkk2+/− littermates, the Dkk2−/− embryos displayed overall normal X-gal staining but an obvious reduction in a small region located at the nasal region of the upper eyelids (Fig. 4C). The decrease of staining was highly consistent among thirty-four Map3k1ΔKD/+/Dkk2−/− eyes examined, and the intensity was significantly less than that in wild type and Dkk2+/− embryos (Fig. 4D). These results indicate that DKK2 serves as a Wnt inhibitor in specific eyelid regions and its ablation leads to activation of Wnt corresponding to repression of MAP3K1.
Activation of the canonical Wnt pathway attenuates MAP3K1-mediated JNK activity
In the developing eyelids, MAP3K1 expression was predominantly occurring in the eyelid epithelial cells. To evaluate the Wnt-MAP3K1 crosstalk in epithelial cells, we examined HaCaT cells treated with LiCl. Similar to what was observed in fibroblasts, LiCl in a dose-dependent manner decreased the level of MAP3K1 proteins (Fig. 5A).
Figure 5. Activation of the canonical Wnt pathway attenuates MAP3K1-mediated JNK activity.
(A) HaCaT cells were treated for 24 h with various concentrations of LiCl; cell lysates were examined by Western blotting for MAP3K1 and β-actin proteins. The relative MAP3K1 versus β-actin levels were quantified with a UVP Imaging System. (B) Wild type and Map3k1ΔKD/ΔKD mouse embryonic stem cells, or (C) HaCaT cells without or with treatment for 24 h with 100 mM LiCl were treated for 30 min with 20 uM S1P. Cell lysates were examined by Western blotting for phosphorylated-JNK and β-actin, or total JNK.
MAP3K1 is an upstream regulator of the JNK MAPKs, crucial for embryonic eyelid development (Takatori et al., 2008). The endogenous stimuli that activate this pathway is yet to be identified; however, we found in cultured ESCs that sphingosine-1 phosphate (S1P) was a potent activator of the pathway (Fig. 5B). Treatment with S1P significantly induced JNK phosphorylation in wild type cells, but induction was totally abolished in the Map3k1-knockout cells, indicating that MAP3K1 was required to transmit the S1P signal to JNK activation.
To test if Wnt would affect the MAP3K1-JNK pathway activity, we pre-treated HaCaT cells with LiCl followed by S1P stimulation. In contrast to the control cells, where S1P strongly induced JNK phosphorylation, the LiCl pre-treated cells had little if any JNK activation in response to S1P (Fig. 5C). Thus, activation of Wnt leads to not only repression of MAP3K1 expression, but also down-regulation of JNK activity in response to specific stimuli.
Wnt signaling inhibits Map3k1 promoter activity
The canonical Wnt pathway plays a pivotal role in cell fate decisions through the induction of β-catenin nuclear translocation. In the nucleus, β-catenin functions as a transcriptional co-activator that binds to the promoter of target genes and regulates gene transcription. Wnt3a is a prototypical canonical pathway activator (Grigoryan et al., 2008). When tested in HEK293 cells, we found that expression of Wnt3a strongly induced TCF/LEF-luc activity, but failed to activate a luciferase reporter controlled by a mutant β-catenin-binding site (Fig. 6A). We subsequently tested the pMap3k1-Luc plasmids in HEK293 cells and examined luciferase activities. Compared to the controls, Wnt3a co-expression led to a marked 60% reduction of the luciferase activity (Fig. 6B), suggesting that activation of Wnt could directly target the Map3k1 promoter and repress its gene expression.
Figure 6. Wnt signaling inhibits Map3k1 promoter.
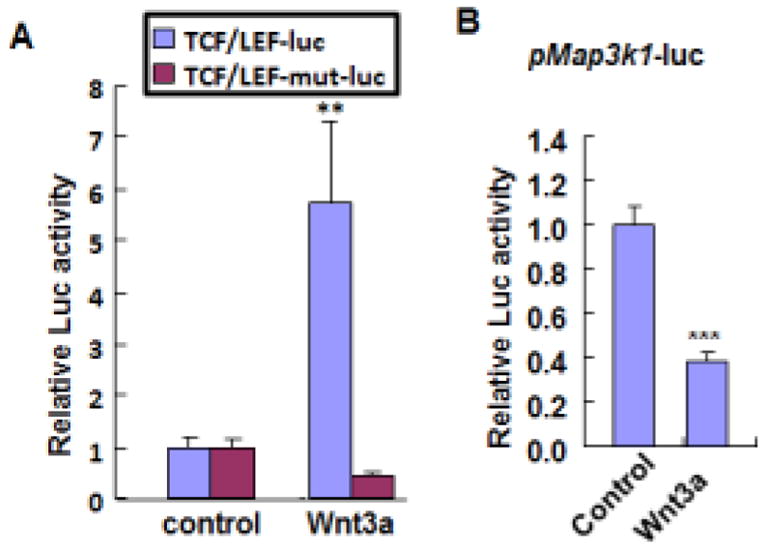
HEK293 cells were co-transfected with CMV-renilla luciferase reporter and (A) Wnt/β-catenin signaling luciferase reporter in its wild type (TCF/LEF-luc) or mutant (TCF/LEF-mut-luc) forms, and (B) Map3k1 promoter driven luciferase reporter (pMap3k1-luc), together with either empty vector or Wnt3a expression plasmids. At 48 h after transfection, luciferase activities were examined. In some experiments, the relative luciferase activity was calculated using firefly-luciferase activity versus renilla-luciferase activity, and the value in control cells was set as 1. The statistical significance was determined using Student t-test, **P<0.01 and ***P<0.001 are considered significant.
Discussion and Conclusion
Using a combination of biochemical and genetic tools with embryonic eyelid development as a model, we identify a unique interaction between Wnt and MAP3K1. We show that activation of Wnt leads to repression of the Map3k1 promoter and hence of gene expression. Decrease of MAP3K1 in turn mitigates JNK MAPKs activation in response to a specific stimulus, i.e. S1P. The crosstalk relationship is detected not only in cultured epithelial cells and fibroblasts, but also in vivo in the embryonic eyelid epithelium. MAP3K1 has been shown to serve as a molecular node, integrating various morphogenetic and environmental signals and connecting different signaling pathways (Geh et al., 2011; Takatori et al., 2008; Zhang et al., 2003). Results described here suggest that the Wnt pathway is an additional arm of this increasingly complex signaling network. It is possible that modulating the MAP3K1-JNK pathways is one of the mechanisms through which Wnt signaling affects eyelid developmental closure.
Wnt signaling is essential for the development of all eye structures. Wnt ligands and their receptors are expressed in the developing retina, lens, cornea and eyelid (Carpenter et al., 2015; Liu et al., 2003). Wnt ligand knockouts display severe ocular abnormalities during development. The Le-Cre mediated ablation of the universal Wnt ligand transporter Wntless in ocular surface ectoderm, for example, leads to severe eye defects that include a saucer-shaped optic cup, ventral coloboma, and a deficiency of periocular mesenchyme (Carpenter et al., 2015). Extensive destruction occurs as early as E11.5, precluding the use of these mice to study the involvement of Wnt in eye morphogenesis at the later development stages. By examining the Axin-LacZ reporter mice, we describe for the first time high level of Wnt activity in the eyelid margin of older embryos, ranging from E14.5–E15.5. These observations provide a circumstantial support for a role of Wnt in the regulation of eyelid closure.
Direct evidence for Wnt’s involvement in eyelid closure comes from studies of the Dkk2 knockout mice. Although not required for embryonic survival and global organogenesis, DKK2 suppresses canonical Wnt signaling locally and transiently during eye development and is required for embryonic eyelid closure (Gage et al., 2008; Meng et al., 2014a; Mukhopadhyay et al., 2006). At E12.5, DKK2 is found to be expressed in the periocular mesenchyme cells and in turn acts in a paracrine fashion to regulate Wnt signaling in epithelial cells located at the migrating front of the eyelid (Gage et al., 2008; Kumar and Duester, 2010). At the developmental stages (E14.5 – E15.5) relevant to the onset of eyelid closure, we find that compared to wild type/Axin-LacZ embryos, the Dkk2−/−/Axin-LacZ embryos do not have overt alterations in Wnt activity, but instead exhibit a slight but significant increase of β-gal activity in a small group of cells located at the upper eyelids in the nasal canthus region. Similar discrete regulation of Wnt activity has been reported before. Using BAT-Gal reporter mice, Wu et. al. have shown that the TCF3–β-catenin axis controls Wnt activity in a band of cells at the mucocutaneous junction between palpebral conjunctiva and palpebral epidermis of the E15.5 embryos (Wu et al., 2012). By examining Axin2 mRNA, Gage et. al. have found that DKK2 suppresses Wnt activity in ocular surface ectoderm, underlying mesenchyme and cornea (Gage et al., 2008). Due to the scope of this paper, we have not looked into the diverse Wnt activity in each eye tissues of the Dkk2−/− embryos, but focused on the developing eyelids. Our data support the idea that DKK2 is one of many Wnt regulators and provides developmental stage-specific suppression of canonical Wnt signaling in distinct eyelid regions. Furthermore, we find that increase of Wnt activity in the Dkk2−/− eyelid corresponds precisely to decrease of MAP3K1 expression. Corroborating with these observations, Wls knockout embryos display elevated MAP3K1 expression near the nasal canthus region. The complementary genetic data strongly support a role of the Wnt pathway in inhibition of MAP3K1 expression in the developing eyelid epithelium.
Wnt-mediated MAP3K1 repression corresponds to down-regulation of Map3k1 promoter activity. The molecular basis underlying promoter repression is not yet understood. Promoter analyses (http://alggen.lsi.upc.es/cgi-bin/promo_v3) have identified a couple of potential TCF/LEF binding sites located at the distal regions approximately -1 kb and -1.7 kb of the Map3k1 promoter. While β-catenin is typically identified as a transcriptional activator, several studies have revealed that it may also act as a transcriptional repressor (Blauwkamp et al., 2008), consistent with a growing body of literature reporting target genes repressed by the Wnt/β-catenin pathway (Hill et al., 2005; Jamora et al., 2003; Kahler and Westendorf, 2003; Leow et al., 2004; Smartt et al., 2012; Spencer et al., 2006). Inhibitory regulation could be mediated through diverse mechanisms, including β-catenin/TCF4 interacting with transcriptional co-repressors (Brantjes et al., 2001), binding to non-canonical TCF4-binding elements (Zhang et al., 2014), or recruiting other co-repressors for a combined repressive effect (Ito et al., 2008; Smartt et al., 2012). Alternatively, repression may occur via TCF4/β-catenin interacting with and inhibiting the transcription factors bound to the Map3k1 promoter, such as AP-1 (Geh et al., 2011; Nateri et al., 2005).
As a stimuli-specific signal transducer, MAP3K1 is required for transmission of the S1P signals to JNK activation. Although MAP3K1 expression is reduced by 35%, S1P-induced JNK activation is completely blocked in LiCl treated cells. These observations suggest there are additional mechanisms through which Wnt may inhibit the MAP3K1-JNK pathway. Indeed, several Wnt negative regulators have been shown to act as positive activators of the MAP3K1-JNK pathway. One of the regulator is Axin, a scaffold protein that facilitates the activation of the MAP3K1-JNK pathway (Zhang et al., 1999), and another is GSK-3β, which directly induces MAP3K1 activity (Kim et al., 2003). Activation of Wnt could compromise the functions of Axin and GSK-3β in MAP3K1 activation. Thus, the complete inhibition of JNK activity by LiCl is possibly the combined consequence of reduction of MAP3K1 expression and activity.
Whereas data shown here suggest that Wnt signaling negatively regulates the MAP3K1 pathway, the reciprocal regulation may also exist. We have previously shown that the activity of the Wnt/β-catenin pathway is higher in MAP3K1 kinase dead cells and developing eyelid epithelium (Jin et al., 2013). In addition, expression of kinase-inactive MAP3K1 {MAP3K1(KM)} significantly elevates TCF/LEF-luc activity but expression of wild type MAP3K1 has minimal effects. One plausible mechanism is that MAP3K1 signaling may lead to the up-regulation of DKK2, which in turn inhibits Wnt activity. The Dkk2 mRNA levels are significantly higher in the eyelid epithelium of wild type embryos relative to that of Map3k1ΔKD/ΔKD embryos (Jin et al., 2013). Hence, MAP3K1 and DKK2 may form a positive regulatory loop to maintain each other’s expression as one of the signaling crosstalk mechanisms in eyelid morphogenesis.
Most of our work focus on the roles of MAP3K1’s kinase activity in the Map3k1ΔKD model, which expresses a C-terminal truncated protein lacking the kinase domain. Besides a kinase domain located at the C-terminus, MAP3K1, being a large protein of 200 kD, possesses several other functional domains. Notably, it has a plant homeodomain (PHD) located at the N-terminus, bearing an E3 ubiquitin ligase activity (Xia et al., 2007). The PHD domain of MAP3K1, interestingly, seems to mediate the activation of the canonical Wnt pathway. Ng, et. al. have shown that through the PHD domain MAP3K1 complexes with Axin1, and this interaction positively regulates Wnt signaling activity and promotes Wnt target gene expression (Sue Ng et al., 2010). The kinase activity of MAP3K1 in contrast is dispensable for Wnt activation. The fact that the different MAP3K1 domains may exhibit distinct or even opposite roles in signal transduction underscores the multifaceted nature of this protein and stresses that the biological functions of MAP3K1 need to be assessed in a context-specific manner.
Work presented here is based primarily on the embryonic eyelid as a model of developmental morphogenesis. The model is relatively easy to study, and like all developmental systems, requires highly coordinated, tightly controlled and exceedingly complex signaling pathways (Rubinstein et al., 2016; Xia and Karin, 2004). Hence, the molecular mechanisms uncovered here could have broader implications in other developmental processes. In this context, it is worth noting that a crosstalk between MAP3K1 and Wnt has recently been found in patients with 46, XY disorder of sex development (Loke et al., 2014). Specifically, the MAP3K1 mutation in patients leads to enhanced Wnt/β-catenin activity. Such signaling crosstalk may also play a significant role in post-natal physiopathological events concerning morphological change and remodeling, such as wound healing and cancer metastasis.
Highlights.
The canonical Wnt pathway is spatiotemporally activated in the embryonic eyelid, and its inhibition leads to increase of MAP3K1 expression.
Genetic removal of the Wnt inhibitor DKK2 results in up-regulation of Wnt activity, corresponding to down-regulation of MAP3K1 expression in a subgroup of eyelid cells.
Activation of Wnt signaling in vitro attenuates Map3k1 promoter activity and reduces the level of MAP3K1 protein.
Activation of Wnt signaling mitigates MAP3K1-mediated activation of the JNK MAPKs.
Acknowledgments
We thank Drs. Richard Lang (Cincinnati Children’s Hospital), Dianqing Wu (Yale University), and Ruth Ashery-Padan (Tel Aviv U) for providing genetic mutant mice, and Dr. Alvaro Puga (U. of Cincinnati) for critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health EY15227 (YX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barishak YR. Embryology of the eye and its adnexae. Dev Ophthalmol. 1992;24:1–142. [PubMed] [Google Scholar]
- Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Berkowitz EA, Seroogy KB, Schroeder JA, Russell WE, Evans EP, Riedel RF, Phillips HK, Harrison CA, Lee DC, Luetteke NC. Characterization of the mouse transforming growth factor alpha gene: its expression during eyelid development and in waved 1 tissues. Cell Growth Differ. 1996;7:1271–1282. [PubMed] [Google Scholar]
- Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008;27:1436–1446. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantjes H, Roose J, van De Wetering M, Clevers H. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 2001;29:1410–1419. doi: 10.1093/nar/29.7.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutation. J Biol Chem. 2002;277:33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Smith AN, Wagner H, Cohen-Tayar Y, Rao S, Wallace V, Ashery-Padan R, Lang RA. Wnt ligands from the embryonic surface ectoderm regulate ‘bimetallic strip’ optic cup morphogenesis in mouse. Development. 2015;142:972–982. doi: 10.1242/dev.120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Zhang X. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet. 2017;33:677–702. doi: 10.1016/j.tig.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, Rosenberg KI. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317:310–324. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geh E, Meng Q, Mongan M, Wang J, Takatori A, Zheng Y, Puga A, Lang RA, Xia Y. Mitogen-activated protein kinase kinase kinase 1 (MAP3K1) integrates developmental signals for eyelid closure. Proc Natl Acad Sci U S A. 2011;108:17349–17354. doi: 10.1073/pnas.1102297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Yuan Z, Ru J, Gu X, Zhang W, Mao F, Ouyang H, Wu K, Liu Y, Liu C. A Spatiotemporal Requirement for Prickle 1-Mediated PCP Signaling in Eyelid Morphogenesis and Homeostasis. Invest Ophthalmol Vis Sci. 2018;59:952–966. doi: 10.1167/iovs.17-22947. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Eyelid development and fusion induced by cortisone treatment in mutant, lidgap-Miller, foetal mice. A scanning electron microscope study. J Embryol Exp Morphol. 1986;91:1–18. [PubMed] [Google Scholar]
- Heller E, Kumar KV, Grill SW, Fuchs E. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev Cell. 2014;28:617–632. doi: 10.1016/j.devcel.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Huang J, Dattilo LK, Rajagopal R, Liu Y, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development. 2009;136:1741–1750. doi: 10.1242/dev.034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, Fukamachi H, Ito Y. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell. 2008;14:226–237. doi: 10.1016/j.ccr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Chen J, Meng Q, Carreira V, Tam NN, Geh E, Karyala S, Ho SM, Zhou X, Medvedovic M, Xia Y. Deciphering gene expression program of MAP3K1 in mouse eyelid morphogenesis. Dev Biol. 2013;374:96–107. doi: 10.1016/j.ydbio.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J Biol Chem. 2003;278:11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee JE, Kim MJ, Cho EG, Cho SG, Choi EJ. Glycogen synthase kinase 3 beta is a natural activator of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) J Biol Chem. 2003;278:13995–14001. doi: 10.1074/jbc.M300253200. [DOI] [PubMed] [Google Scholar]
- Kumar S, Duester G. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol. 2010;340:67–74. doi: 10.1016/j.ydbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Baek RM, Chung WJ. Nonincisional blepharoplasty using the debulking method. Aesthetic Plast Surg. 2003;27:434–437. doi: 10.1007/s00266-003-3096-9. [DOI] [PubMed] [Google Scholar]
- Leow CC, Romero MS, Ross S, Polakis P, Gao WQ. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64:6050–6057. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Loke J, Pearlman A, Radi O, Zuffardi O, Giussani U, Pallotta R, Camerino G, Ostrer H. Mutations in MAP3K1 tilt the balance from SOX9/FGF9 to WNT/beta-catenin signaling. Hum Mol Genet. 2014;23:1073–1083. doi: 10.1093/hmg/ddt502. [DOI] [PubMed] [Google Scholar]
- Meng Q, Mongan M, Carreira V, Kurita H, Liu CY, Kao WW, Xia Y. Eyelid closure in embryogenesis is required for ocular adnexa development. Invest Ophthalmol Vis Sci. 2014a;55:7652–7661. doi: 10.1167/iovs.14-15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Mongan M, Wang J, Tang X, Zhang J, Kao W, Xia Y. Epithelial sheet movement requires the cooperation of c-Jun and MAP3K1. Dev Biol. 2014b;395:29–37. doi: 10.1016/j.ydbio.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine N, Iwamoto R, Mekada E. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005;132:4317–4326. doi: 10.1242/dev.02030. [DOI] [PubMed] [Google Scholar]
- Mongan M, Meng Q, Wang J, Kao WW, Puga A, Xia Y. Gene-Environment Interactions Target Mitogen-activated Protein 3 Kinase 1 (MAP3K1) Signaling in Eyelid Morphogenesis. J Biol Chem. 2015;290:19770–19779. doi: 10.1074/jbc.M115.665729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Higashiyama S, Mitamura T, Mekada E, Taniguchi N. Amino-terminal processing of cell surface heparin-binding epidermal growth factor-like growth factor up-regulates its juxtacrine but not its paracrine growth factor activity. J Biol Chem. 1996;271:30858–30863. doi: 10.1074/jbc.271.48.30858. [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Rubinstein TJ, Weber AC, Traboulsi EI. Molecular biology and genetics of embryonic eyelid development. Ophthalmic Genet. 2016;37:252–259. doi: 10.3109/13816810.2015.1071409. [DOI] [PubMed] [Google Scholar]
- Smartt HJ, Greenhough A, Ordonez-Moran P, Talero E, Cherry CA, Wallam CA, Parry L, Al Kharusi M, Roberts HR, Mariadason JM, Clarke AR, Huelsken J, Williams AC, Paraskeva C. beta-catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut. 2012;61:1306–1314. doi: 10.1136/gutjnl-2011-300817. [DOI] [PubMed] [Google Scholar]
- Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- Sue Ng S, Mahmoudi T, Li VS, Hatzis P, Boersema PJ, Mohammed S, Heck AJ, Clevers H. MAP3K1 functionally interacts with Axin1 in the canonical Wnt signalling pathway. Biol Chem. 2010;391:171–180. doi: 10.1515/bc.2010.028. [DOI] [PubMed] [Google Scholar]
- Takatori A, Geh E, Chen L, Zhang L, Meller J, Xia Y. Differential transmission of MEKK1 morphogenetic signals by JNK1 and JNK2. Development. 2008;135:23–32. doi: 10.1242/dev.007120. [DOI] [PubMed] [Google Scholar]
- Tao H, Ono K, Kurose H, Noji S, Ohuchi H. Exogenous FGF10 can rescue an eye-open at birth phenotype of Fgf10-null mice by activating activin and TGFalpha-EGFR signaling. Dev Growth Differ. 2006;48:339–346. doi: 10.1111/j.1440-169X.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Uhlik MT, Abell AN, Cuevas BD, Nakamura K, Johnson GL. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol. 2004;82:658–663. doi: 10.1139/o04-114. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CI, Hoffman JA, Shy BR, Ford EM, Fuchs E, Nguyen H, Merrill BJ. Function of Wnt/beta-catenin in counteracting Tcf3 repression through the Tcf3-beta-catenin interaction. Development. 2012;139:2118–2129. doi: 10.1242/dev.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Xia Y, Makris C, Su B, Li E, Yang J, Nemerow GR, Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc Natl Acad Sci U S A. 2000;97:5243–5248. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wang J, Xu S, Johnson GL, Hunter T, Lu Z. MEKK1 mediates the ubiquitination and degradation of c-Jun in response to osmotic stress. Mol Cell Biol. 2007;27:510–517. doi: 10.1128/MCB.01355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger GR, Henson P, Johnson GL. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc Natl Acad Sci U S A. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CU, Blauwkamp TA, Burby PE, Cadigan KM. Wnt-mediated repression via bipartite DNA recognition by TCF in the Drosophila hematopoietic system. PLoS Genet. 2014;10:e1004509. doi: 10.1371/journal.pgen.1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang W, Hayashi Y, Jester JV, Birk DE, Gao M, Liu CY, Kao WW, Karin M, Xia Y. A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure. EMBO J. 2003;22:4443–4454. doi: 10.1093/emboj/cdg440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Neo SY, Wang X, Han J, Lin SC. Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem. 1999;274:35247–35254. doi: 10.1074/jbc.274.49.35247. [DOI] [PubMed] [Google Scholar]