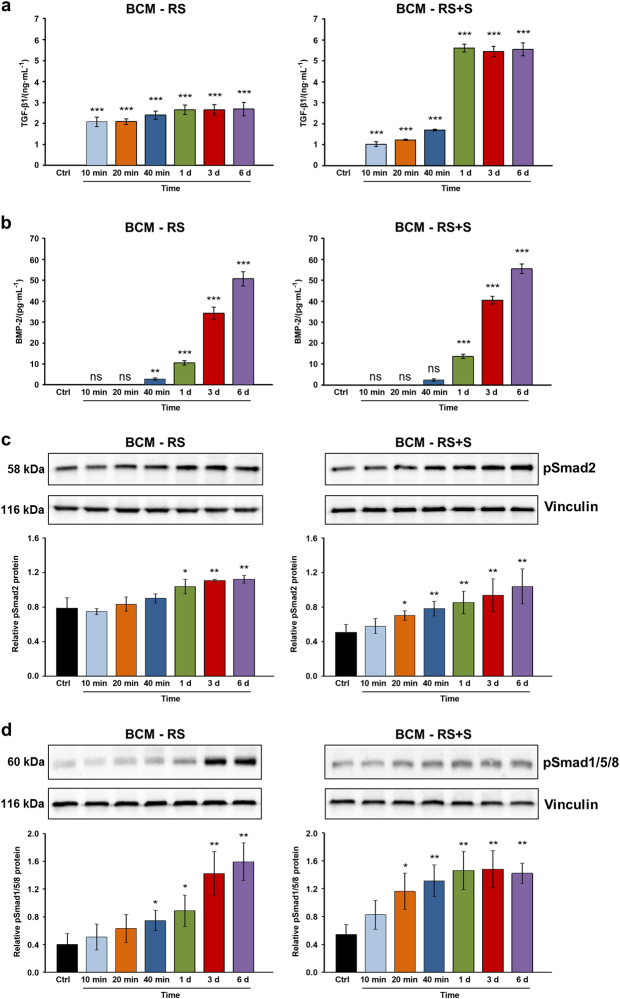
Fig. 1.
Release of TGF-β1 and BMP-2 proteins from the cortical bone and the induction of Smad signaling in mesenchymal stromal ST2 cells. a, b ELISA quantification of TGF-β1 (a) and BMP-2 (b) proteins contained in bone-conditioned medium extracted from the cortical bone chips with either Ringer’s solution (BCM-RS) or Ringer’s solution mixed with autologous serum (BCM-RS+S) at a 1:1 ratio. The two types of media were collected at 10, 20, and 40 min, as well as at 1, 3, and 6 d. Controls (ctrl) represent RS or RS+S, not containing bone particles (controls exhibited no detectable levels of the two proteins tested). Mean±standard deviations represent three independent BCM preparations, and significant differences to the respective controls (***P < 0.001, **P < 0.01, ns non-significant) are shown. c, d BCM induces enhanced phosphorylation of TGF-β1- and BMP-2-specific R-Smads in mesenchymal stromal ST2 cells. Immunoblot analyses of phospho-Smad2 (pSmad2) (c) and phospho-Smad1/5/8 (pSmad1/5/8) (d) proteins in whole-cell extracts from ST2 cells treated with BCM-RS or BCM-RS+S used in a, b. The bar charts represent densitometric quantification of the immunoblots. pSmad2 and pSmad1/5/8 levels are normalized to vinculin loading controls. Data represent mean±SD obtained from three independent experiments. Significant differences to the control cells treated with RS or RS+S (**P < 0.01, *P < 0.05) are shown