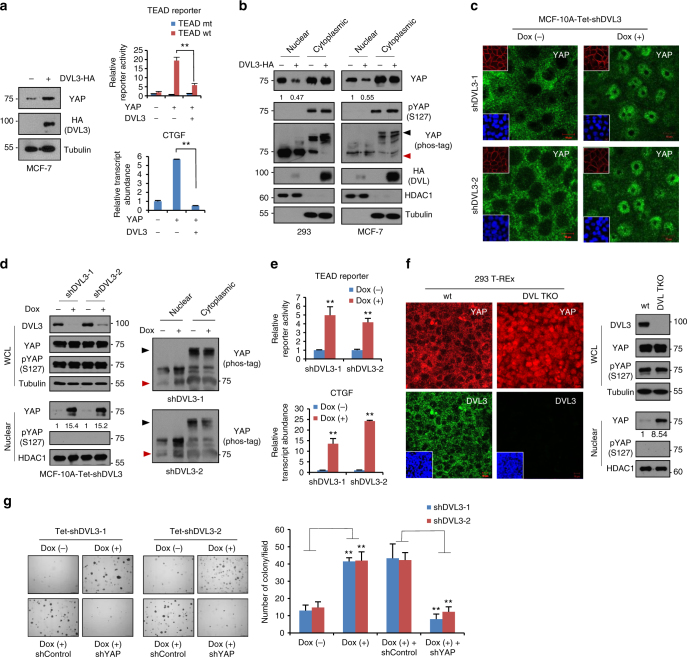
Fig. 2.
DVL suppresses YAP nuclear abundance and TEAD transcriptional activity. a YAP was co-transfected with HA-DVL3 into MCF-7 cells, and protein abundance (left), TEAD reporter activity (right upper), and CTGF transcript abundance (right lower) were determined. Data of reporter assay and RT-PCR are normalized to negative control empty vector (−) and presented as mean ± SD. b The 293 and MCF-7 cells were transfected with DVL3, and protein abundance of YAP in nuclear and cytoplasmic fraction was determined by immunoblot analysis. YAP phosphorylation status in cytoplasmic and nuclear fraction was determined by pS127-YAP antibody and mobility shift on a phos-tag gel. Red arrowhead indicates active YAP on a phos-tag gel. Tubulin and HDAC1 served as loading controls of cytosolic fraction and nuclear lysates, respectively. Relative nuclear YAP abundance compared to control was measured by ImageJ. c, d Inducible knockdown of DVL3 increases nuclear YAP abundance. The MCF-10A cells expressing tetracycline-inducible shRNA against DVL3 were generated with lentiviral system, and endogenous YAP localization and abundance without or with doxycycline (Dox) were determined by immunofluorescence (c) and immunoblot analysis from whole-cell lysate (WCL) and nuclear fraction (d). The cells were serum-starved for 16 h before harvest. Tubulin and HDAC1 served as loading controls of cytosolic fraction and nuclear lysates, respectively. e DVL3 was knockdowned with doxycycline (Dox), and the TEAD reporter activity (upper panel) and CTGF transcript abundance (lower panel) were determined by reporter assay and qRT-PCR, respectively. f The wt and DVL-TKO cells were cultured in confluent condition and subcellular localization of endogenous YAP and DVL3 were determined by confocal microscopy (left panels) and immunoblot analysis (right panels). The cells were serum-starved for 16 h before examination. Inset, DAPI nuclear stain; Scale bar, 10 μm. g Knockdown of DVL3 increases anchorage-independent growth of MCF-7 cells. The MCF-7 cells expressing inducible shRNA for DVL3 were seeded onto a soft agar without (Dox-) or with (Dox+) doxycycline in combination with shControl or shYAP for 3 weeks. The colonies were stained with crystal violet and quantified. Data presented as mean ± SD, n = 5