Fig. 3.
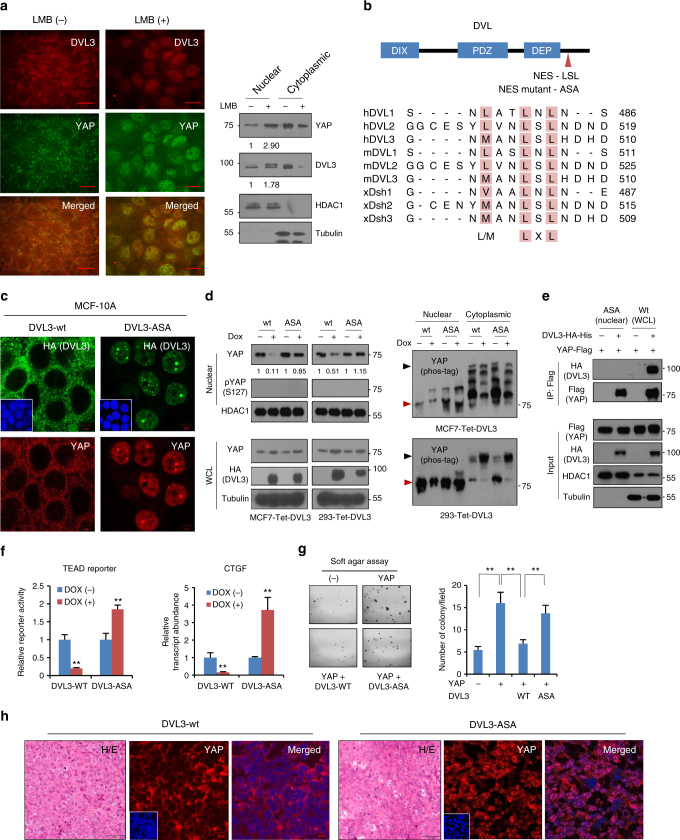
NES (nuclear export sequence) in DVL is responsible for YAP trafficking. a The confluent MCF-7 cells were treated with Leptomycin B (LMB, 5 ng ml−1) for 4 h, and endogenous YAP and DVL localization and abundance were determined by confocal microscopy (left panels) and immunoblotting (right panels). Scale bar, 10 μm. b Schematic representation of the conserved NES and point mutant (NES-mutant-ASA) in DVL of human (h), mouse (m), and xenopus (x). c The MCF-7 cells were transfected with wt or NES-ASA mutant DVL3 and DVL-YAP localization was determined by confocal microscopy. The cells were serum-starved for 16 h before harvest. Scale bar, 5 μm. d MCF-7 and 293 cells expressing inducible HA-tagged wt or NES-mutant (ASA) of DVL3 were treated with doxycycline, and YAP and DVL abundance were determined (left panels). YAP phosphorylation status in cytoplasmic and nuclear fraction was determined by pS127-YAP antibody and mobility shift on a phos-tag gel (right panels). Red arrowhead indicates active YAP. e The 293 cells were transfected with flag-tagged YAP with NES-mutant or wt DVL3. The nuclear protein form NES-mutant transfectant was subjected for immunoprecipitation, whole-cell lysate of wt DVL serving as control. Fifty micro grams of nuclear protein and 10 μg of whole-cell lysates were used for immunoprecipitation assay to adjust YAP abundance. f TEAD reporter activity and CTGF transcript abundance were analyzed from MCF-7 cells expressing inducible wt or NES-mutant of DVL3 (mean ± SD, n = 3). g The MCF-7 cells were transfected with YAP in combination with wt or NES-ASA mutant of DVL3, then seeded onto a soft agar for 3 weeks. The colonies were stained with crystal violet and quantified (mean ± SD, n = 5). h The 293 cells stably expressing tet-inducible wt or NES-mutant of DVL were injected into athymic nude mice subcutaneously. When the tumor volume reached around 500 mm3 (n = 1), the mice were treated with doxycycline (50 mg/kg) intraperitoneally prior sacrifice to 24 h. The tissues were examined by H/E staining (scale bar, 50 μm) and YAP localization was determined from frozen sections (scale bar, 10 μm). Unprocessed original scans of blots are shown in Supplementary Fig. 10