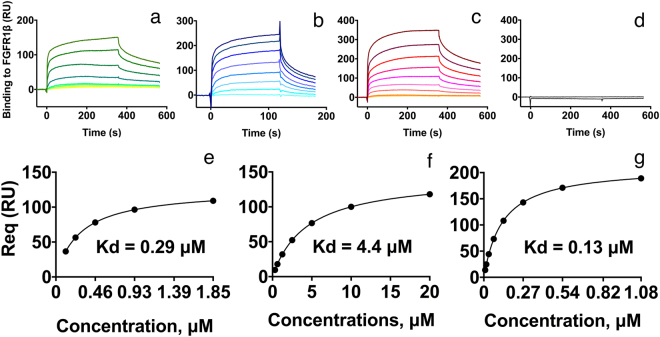
Figure 3.
NCAM2 FnIII domains bind to FGFR1. Surface plasmon resonance analysis was utilized to investigate interactions between the NCAM2 FnIII1, FnIII2, and FnIII1-2 domains and the ectodomain of FGFR1. Approximately 1000 resonance units (RUs) of the FGFR1β ectodomain (Ig2-Ig3) were immobilized on a sensor chip and recombinant NCAM2 FnIII1 (a), FnIII2 (b) and FnIII1-2 (c) were injected at the indicated concentrations. Binding is expressed as the response difference between the binding of the respective recombinant proteins to a cell on the sensor chip with the immobilized FGFR1β and a control cell on the same sensor chip coated with IgG (d). On the basis of the presented steady state affinity fit curves, Kd values of 0.29 (χ2 = 0.057), 4.4 (χ2 = 0.016) and 0.13 (χ2 = 0.028) μM were estimated for NCAM2 FnIII1 (d), FnIII2 (e) and FnIII1-2 (f), respectively.