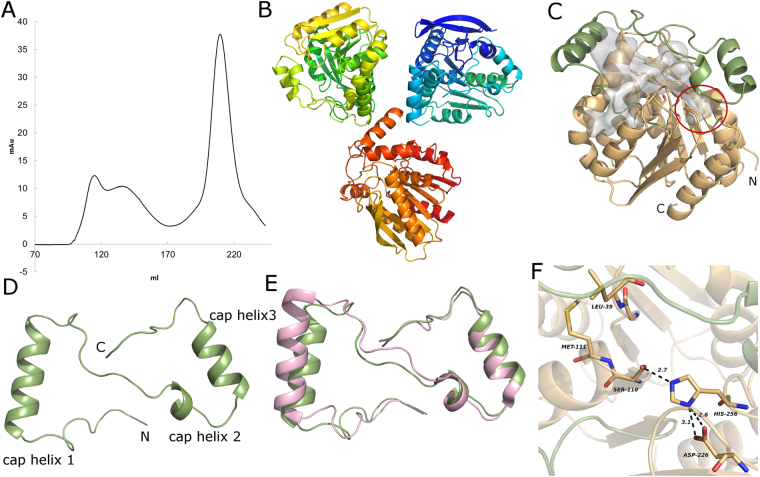
Figure 1.
(A) Size exclusion chromatogram of mtbMGL after purification via Ni-NTA Agarose resin. The monomeric fraction (eluting at approximately 205 ml) was used for crystallization. (B) Asymmetric unit in the mtbMGL crystal. (C) Overall structure of mtbMGL (chain B), the cap region is in green and the α/β hydrolase core in wheat. The gray blob represents the cavity inside the protein calculated by CASOX. The small cavity accommodating the glycerol moiety is marked with a red circle. (D) Cap region of mtbMGL viewed from the top (compared to orientation in 1C). (E) Cap regions from hMGL and mtbMGL aligned to each other (hMGL in pink, mtbMGL in green. (F) Close up view on the active site of mtbMGL with the residues of the catalytic triad and the residues that build up the oxyanion hole shown as sticks.