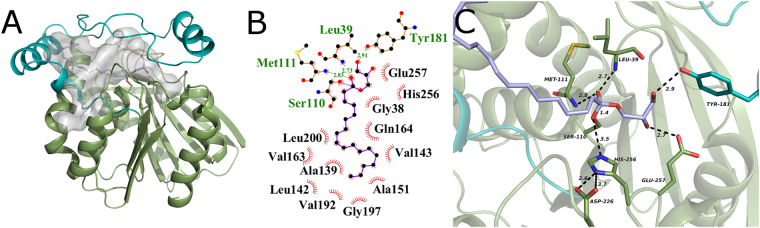
Figure 2.
(A) Cartoon representation of the mtbMGL crystal structure with 1-OG docked. The cap is shown in cyan and the α/β hydrolase core in green. The gray blob shows the cavity inside the protein calculated omitting the ligand. Gray sticks show 1-OG covalently bound to the active site serine. (B) Ligplot+48 cartoon of all the residues interacting with the ligand. The hydrophobic interactions are shown as brown circle sections. (C) Close up view of catalytic triad and residues interacting with the glycerol moiety in the 1-OG docked mtbMGL crystal structure. Residues interacting with the glycerol moiety (Tyr181, Glu257) are shown as sticks.