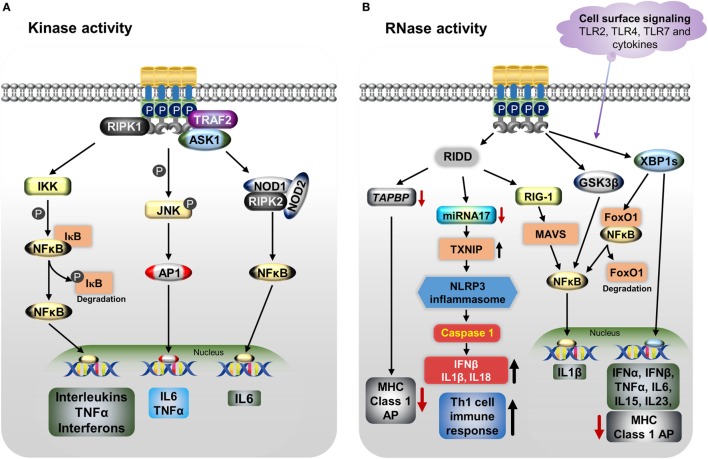
Figure 2.
IRE1α regulates the immune function through various mechanisms including both its kinase (A) and RNase (B) functions. (A) Endoplasmic reticulum (ER) stress-activated, serine/threonine-protein kinase/endoribonuclease IRE1α binds to TNF receptor-associated factor 2 (TRAF2), apoptosis signaling kinase1 (ASK1), and receptor-interacting serine/threonine protein kinase 1 (RIPK1), resulting in phosphorylation of c-Jun N-terminal kinase. Then c-Jun interacts with c-Fos forms the active transcription factor AP-1, and increases the production of IL-6 and TNFα. Furthermore, the IRE1α/TRAF2/ASK1 complex activates the inhibitory kappa B kinase (IKK), which phosphorylates inhibitor of kappa B (IκB), leading to release of NFκB and its translocation to the nucleus, where it induces the expression of cytokines. The dissociated IκB is then degraded by proteasomes. The IRE1α–TRAF2 complex increases IL-6 production via the association of nucleotide-binding oligomerization domain (NOD)-containing proteins 1 and 2 (NOD1 and NOD2) and receptor-interacting serine/threonine-protein kinase 2 (RIPK2). (B) IRE1α through its RNase function generates splices—X-box-binding protein 1 (XBP1s) transcription factor induces the expression of several pro-inflammatory cytokines and also decreases MHC class I antigen presentation. In addition, XBP1s increases NFκB nuclear translocation by mediating the degradation of FoxO1, an inhibitor of NFκB. Furthermore, IRE1α activation differentially regulates the expression of the pro-inflammatory cytokine IL-1β gene via activation of glycogen synthase kinase-3β. The regulated IRE1α-dependent decay (RIDD) degrades miR-17, leading to an increase in thioredoxin-interacting protein expression. This in turn activates nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasome activity, which leads to procaspase-1 cleavage, which subsequently activates IL-1β, IL-18, and IFN-β, and also increases the Th-cell 1 immune response. RIDD generates small fragments of RNA, which activate the retinoic inducible gene-I and mitochondrial antiviral protein, increasing IFNβ production via NFκB. In addition, RIDD reduces the TAPBP mRNA level, leading to decreased antigen presentation. Toll-like receptors 2, 4, and 7 and other cytokines can directly activate the IRE1α/XBP1s pathway without ER stress and cause the release of many pro-inflammatory cytokines.