Abstract
Background
Several tumor entities including brain tumors aberrantly overexpress intermediate conductance Ca2+ activated KCa3.1 K+ channels. These channels contribute significantly to the transformed phenotype of the tumor cells.
Method
PubMed was searched in order to summarize our current knowledge on the molecular signaling upstream and downstream and the effector functions of KCa3.1 channel activity in tumor cells in general and in glioblastoma cells in particular. In addition, KCa3.1 expression and function for repair of DNA double strand breaks was determined experimentally in primary glioblastoma cultures in dependence on the abundance of proneural and mesenchymal stem cell markers.
Results
By modulating membrane potential, cell volume, Ca2+ signals and the respiratory chain, KCa3.1 channels in both, plasma and inner mitochondrial membrane, have been demonstrated to regulate many cellular processes such as migration and tissue invasion, metastasis, cell cycle progression, oxygen consumption and metabolism, DNA damage response and cell death of cancer cells. Moreover, KCa3.1 channels have been shown to crucially contribute to resistance against radiotherapy. Futhermore, the original in vitro data on KCa3.1 channel expression in subtypes of glioblastoma stem(-like) cells propose KCa3.1 as marker for the mesenchymal subgroup of cancer stem cells and suggest that KCa3.1 contributes to the therapy resistance of mesenchymal glioblastoma stem cells.
Conclusion
The data suggest KCa3.1 channel targeting in combination with radiotherapy as promising new tool to eradicate therapy-resistant mesenchymal glioblastoma stem cells.
Keywords: SK4, IKCa, KCNN4, GBM, GSCs, ALDH1A3, γH2AX foci, radioresistance
1. Introduction
KCa3.1 (SK4, IKCa, KCNN4) is a Ca2+-activated K+ channel (for review on the biophysics of IKCa see [1] in this special issue on KCa3.1 channels and glioblastoma) which was first described functionally more than half a century ago by the Hungarian physiologist György Gárdos [2] who identified KCa3.1 as the K+ conductance in human red blood cells that mediates the efflux of KCl and isoosmotically obliged H2O together with a Cl- permeability and aquaporines. The resulting red blood cell shrinkage is paralleled by the morphological transition of the discoid cells into echinocytes. Meanwhile, several physiological and patho-physiological functions have been attributed to the erythrocytic KCa3.1 channel. Among those are induction of red blood cell apoptosis (referred to as eryptosis) [3, 4] and irreversible sickling of homozygous HbS/HbS erythrocytes [5]. Therefore, KCa3.1 channel inhibitors such as senicapoc have been tested in clinical trials for their capability to prevent crises of sickle cell anemia patients. These and further trials showed that senicapoc accumulated in the plasma to effective plasma concentrations regarding KCa3.1 channel inhibition and that senicapoc was well tolerated indicating that KCa3.1 channels are druggable therapy targets [6] in this special issue on KCa3.1 channels and glioblastoma.
In addition to erythrocytes, a variety of cell types in different organs express KCa3.1 channels. For instance, in epithelial cells of the colon, KCa3.1 contributes to transepithelial fluid secretion [7]. Other cell types include vascular smooth muscle and endothelial cells where KCa3.1 regulates vasodilatation [8] or Na+ transport across the blood brain barrier [9]. Beyond the classical physiological functions, K+ channels have been identified to act within biochemical signaling cascades (for review see [10]). As an example, K+ channels transduce insulin-like growth factor (IGF) signals downstream from IGF receptor, phosphatidylinositide 3-kinase (PI3) kinase, and serum- and glucocorticoid-dependent kinases (SGKs) [11, 12] via enhancing store-operated Ca2+ entry as prerequisite for the activation of downstream Ca2+ effector proteins that contribute to mitogenic signaling. Mechanistically, activated SGKs attenuate removal of K+ channels from the plasma membrane and, hence, increase their surface expression. Enhanced activity of plasmalemmal K+ channels in turn, is required to stabilize the membrane potential and to maintain the electrochemical driving force for Ca2+ [13]. Along those lines, proliferation of activated T lymphocytes has been demonstrated to depend critically on KCa3.1 activity suggesting that KCa3.1 electrosignaling is a regulatory element of the adaptive immune system [14]. Notably, KCa3.1 functions also in brain tumor-associated microglia pointing to an immunomodulating effect of any KCa3.1-targeting therapy [15] in this special issue on KCa3.1 channels and glioblastoma.
In addition to mitogenic signaling, KCa3.1 channels in the inner mitochondrial membrane have been proposed to modulate mitochondrial function in colon cancer cells [16, 17]. In pancreatic cancer cells, mitochondrial KCa3.1 channels have been suggested to regulate oxidative phosphorylation [18]. Likewise, an adapting function in metabolism has been attributed to plasmalemmal KCa3.1 channels. In genotoxically stressed lung adenocarcinoma cells, KCa3.1 channel activation [19] parallels increase in glucose uptake by Na+-coupled cotransport [20, 21] suggesting that KCa3.1 increases/sustains the driving force for Na+-coupled glucose fueling. In addition, mitochondrial KCa3.1 channels have been proposed to contribute to apoptosis induction in melanoma cells [22, 23]. Plasmalemmal KCa3.1 channels have been identified in human D54 glioblastoma cells to contribute to apoptotic cell volume decrease triggered by the intrinsic but not extrinsic pathway [24]. In summary, these studies indicate the functional significance of KCa3.1 in key cell biological and metabolic processes. The present article aims to review the published in vitro data on KCa3.1 function in tumors and in particular in glioblastoma cells. Beyond that, this article provides original data on the role of KCa3.1 in therapy resistance of glioblastoma stem cells.
1.1. KCa3.1 Channels in Tumor Cells: Activation by Ionizing Radiation
Several tumor entities have been demonstrated to up-regulate KCa3.1 channels. Among those are breast [25], lung [26, 27], pancreatic [28], prostate cancer [29, 30], T cell leukemia [31] as well as glioblastoma [32, 33]. KCa3.1 channels reportedly exert oncogenic functions and contribute to neoplastic transformation [25], cell proliferation [28, 29], tumor spreading [34-36] and resistance to chemo- and radiotherapy [31, 37, 38]. In particular in glioblastoma cells, ionizing radiation has been shown in vitro to induce KCa3.1 channel activity probably via radiation-stimulated stabilization of HIF-1α, upregulation of the HIF-1α target gene stromal-cell-derived factor-1 (SDF1; CXCL12), auto-/paracrine SDF-1 signaling via its chemokine receptor CXCR4 [39, 40], and consecutive Ca2+ store release and store-operated Ca2+ entry [40]. Radiogenic stabilization of HIF-1α has been suggested to occur either directly by S-nitrosylation [41] or indirectly via radiogenic phospholipid peroxidation-mediated activation of the EGF receptor [42] and subsequent translocation of the receptor to the nucleus. Nuclear EGF receptor, in turn, has been proposed to facilitate HIF-1α signaling [43].
1.2. KCa3.1 Channels Confer Therapy Resistance to Glioblastoma Cells
Radiogenic KCa3.1 channel activity modifies the Ca2+ signaling in glioblastoma cell lines. This is evident from the observation that the KCa3.1 channel inhibitor TRAM-34 decreased steady state free cytosolic Ca2+ concentration or triggered Ca2+ oscillations in irradiated glioblastoma cells [10]. The latter suggests that Ca2+ oscillations may be inhibited by KCa3.1 activity as has been predicted for highly hormone-stimulated cells by a theoretical model on the role of Ca2+-activated K+ channels in the regulation of hormone-induced Ca2+ oscillations [44]. Together, this hints to a reciprocal interaction between Ca2+- release and entry pathways on the one hand and KCa3.1 channels on the other.
Ca2+ signals reportedly regulate cell cycle progression via Ca2+ effector proteins such as Ca2+/calmodulin-dependent kinases-II (CaMKIIs) [45]. In glioblastoma cells, ionizing radiation has been demonstrated in vitro to induce Ca2+ signals [38] and to activate CaMKIIs in a K+ channel-dependent manner [10, 40, 46]. In other tumor entities, such radiogenic CaMKII activity has been demonstrated to contribute critically to G2/M cell cycle arrest by inactivation of the phosphatase cdc25B. Inactivation of cdc25B results in maintenance of cdc2 (cyclin-dependent kinase-1, CDK1) in its phosphorylated, inactive form [31, 47, 48]. Arresting the cell cycle is crucial for repair of DNA damages, in particular of DNA double strand breaks. Entry into mitosis with residual DNA double strand breaks leads to chromosome aberrations eventually resulting in cell death (mitotic catastrophe).
1.3. KCa3.1 Channels Control Cell Cycling in Glioblastoma Cells
In a previous study of our group, pharmacological inhibition or knockdown of KCa3.1 impaired cell cycle control and G2/M cell cycle arrest in irradiated but not in control T98G glioblastoma cells, suggesting a specific function of KCa3.1 in DNA damage response [38]. Accordingly, targeting of KCa3.1 with TRAM-34 increased the number of residual γH2AX foci as a surrogate marker of unrepaired, residual DNA double strand breaks in that study [38]. In parallel, TRAM-34 attenuated clonogenic survival of irradiated parental and Mock-transduced T98G cells as well as of U-87MG glioblastoma cells. In contrast, a T98G clone stably expressing KCa3.1 shRNA was not radiosensitized by TRAM-34 and exhibited a lower radioresistance than the Mock-transduced T98G cells [38]. Vice versa, experimental up-regulation of KCa3.1 expression in U251 glioblastoma cells conferred radioresistance and rendered the cells sensitive to TRAM-34 in colony formation assays [10]. Similarly, TRAM-34 has been shown to force G2/M cell cycle progression in GL261 glioma cells treated with the DNA-alkylating drug temozolomide probably through cdc2 de-phosphorylation which facilitates apoptotic cell death and impairs clonogenic survival [37]. Combined, these data clearly indicate a radio- and chemotherapy-protecting function of KCa3.1 in glioblastoma cells. Moreover, these results demonstrate that KCa3.1 may act as integrated signaling module within biochemical signal transduction pathways.
1.4. KCa3.1 Channels Foster Brain Infiltration of Glioblastoma Cells
Beyond electrosignaling in cell cycle control, KCa3.1 plays a pivotal role in the programming and/or mechanics of cell migration and invasion into the brain parenchyma [32, 34, 36, 37, 49] (for review also see [15] in this special issue on KCa3.1 channels and glioblastoma). Cell migration is motorized by volume decrease at the cell rear and volume expansion of the invadipodium that result in a directed movement along the rear/invadipodium axis. Volume changes on both cell poles are generated by net uptake/efflux of electrolytes which is accompanied by concordant fluxes of isoosmotically obliged H2O [50]. Glioblastoma cells, in particular, invade the brain parenchyma by migration along blood vessels and neurofibres using them as tracks. By doing so, glioblastoma cells have to squeeze through narrow interstitial spaces which requires highly efficient local cell volume control [51].
Functional KCa3.1 channel expression has been demonstrated by patch-clamp on-cell recording at the cell rear of T98G, U-87MG [38], and U251 cells [10]. In addition, immunohistochemistry data indicate KCa3.1 channel protein expression at the invadipodium of D54 human glioma cells [36]. The KCa3.1 channel blocker TRAM-34 reportedly inhibits both, fetal calf serum (FCS)-induced Ca2+ oscillation and FCS-stimulated chemotaxis in transwell migration assays in a subpopulation of U-87MG glioblastoma cells [32]. Similarly, TRAM-34 inhibits bradykinin-induced chemotaxis of human D54 glioma cells [36]. In addition to cell migration, conditional KCa3.1 knockdown and/or TRAM-34 reportedly inhibit the in situ invasion of U251 cells and primary glioblastoma neurosphere cultures into brain slices [34]. Likewise, TRAM-34 has been documented to inhibit migration and invasion of GL261 glioma cells in wound healing and Matrigel invasion assays, respectively [37]. Taken together, these in vitro experiments clearly indicate the functional significance of KCa3.1 channels for glioblastoma cell migration and brain invasion.
1.5. KCa3.1 Channels are Upregulated in Glioblastoma Stem Cells
Glioblastoma stem-like cells have been proposed to constitute the most therapy-resistant and most invasive subpopulation of glioblastoma cells responsible for brain infiltration and therapy failure [52-55]. Notably, KCa3.1 channels are overproportionally up-regulated in the CD133 stem cell marker-positive subpopulation of gliobastoma cells as demonstrated in the U-87MG line grown in stem cell-enriched neurospheres and in the 2B5 subclone of FCN9 cells. In stem cell-enriched U-87MG and 2B5 cells, TRAM-34 strongly attenuated invasion as assessed by fibronectin-coated Boyden chamber assay [49] suggesting that migration of glioblastoma stem cells crucially depends on KCa3.1 function. Along those lines, in CD133+ stem cell-enriched primary neurosphere cultures of human glioblastoma specimens, only temozolomide in combination with TRAM-34 was capable to inhibit DNA synthesis as measured by [3H]-thymidine incorporation [37]. In addition to KCa3.1 function in cancer stem cells, KCa3.1 activity has been reported in normal mesenchymal stem cells [56, 57] and neuronal precursor cells [58]. Combined, these data point to a specific KCa3.1 function in normal and glioblastoma stem cells.
1.6. Stem Cells of Molecular Glioblastoma Subtypes
Human glioblastoma are classified in four molecular subgroups referred to as classical, mesenchymal, proneural and neural [59]. Mesenchymal and proneural types of glioblastoma stem cells have been identified which present gene signatures similar to the mesenchymal and the proneural molecular glioblastoma subgroup, respectively. Importantly, mesenchymal stem cells have been suggested in some studies to exhibit a more aggressive phenotype and higher radioresistance than proneural stem cells [60, 61]. Analysis of clinical data from glioblastoma patients, in contrast, suggests, that tumors with mesenchymal expression profiles predict for longer survival than proneural expression profiles [59, 62].
Mesenchymal cancer stem cells have been demonstrated to highly upregulate the mesenchymal aldehyde dehydrogenase isoform 1A3 (ALDH1A3) [60, 63, 64]. Subjecting glioma cells to ionizing radiation induces up- and downregulation of markers associated with mesenchymal and proneural properties, respectively. Most importantly, radiation-induced upregulation of mesenchymal markers depend on ALDH1A3 function [60]. Moreover, in neuroblastoma, ALDH1A3 knock-out reduces clonogenicity and self-renewal capacity [65]. In head and neck squamous cell carcinoma, knock-down of ALDH1A3 decreases radioresistance [66]. In addition, ALDH1A3 downregulation reduces resistance to chemotherapy in malignant pleural mesothelioma cells [67]. Finally, in glioma ALDH1A3 knockdown suppresses the ability of tumor invasion [64]. Together, these data clearly indicate that the aggressiveness of mesenchymal cancer stem cells strongly relies on ALDH1A3-mediated signaling.
1.7. KCa3.1 Channels Contribute to the Malignancy of Mesenchymal Glioblastoma Stem Cells
In addition to reviewing the literature, the present article aims to compare KCa3.1 expression and function between mesenchymal and proneural glioblastoma stem cells. To this end, human resection specimen-derived glioblastoma cells were grown under cancer stem cell-enriching culture conditions and KCa3.1 mRNA abundance was determined in dependence on the expression of stem cell markers. In addition, the functional significance of KCa3.1 for radioresistance was defined by analyzing repair of DNA double strand breaks in dependence on KCa3.1 inhibition.
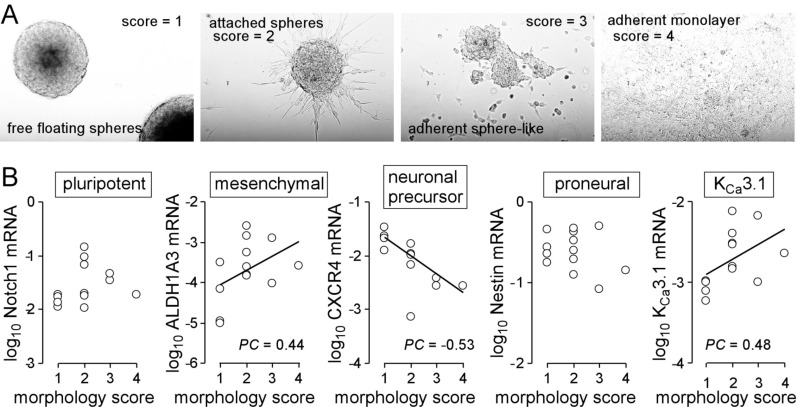
Cell suspension of individual glioblastoma resection specimens (n = 13) grew in stem cell medium either as floating globular spheres, as attached spheres, as sphere-like attached layer, or as adherent monolayer with spotted spheroid-like uprisings (Fig. 1A).
Fig. (1).
Heterogeneity of primary glioblastoma cultures. A, B. Morphological phenotypes of primary glioblastoma cultures (A) and their association (B) with mRNA abundances of stem cell markers and of IKCa3.1 (PC: Pearson correlation coefficient). Resected glioblastoma tissue (patients gave informed consent and this study has been approved by the local ethic committee under project #579/2015BO2) was dissociated enzymatically and mechanically and plated in Complete NeuroCult™ NS-A Proliferation medium containing 20 ng/ml rhEGF, 10 ng/ml rhbFGF and 0.0002% heparin (STEMCELL Technologies Germany GmbH, Cologne, Germany). Every second day 1/10 of the original medium volume was replenished (for RT-PCR, see Legend to Fig. 2).
Scoring these culture phenotypes from 1 (floating spheroids) to 4 (monolayer) and plotting these scores against the mRNA abundances of the pluripotent stem cell marker Notch1 [68], the mesenchymal marker ALDH1A3, the neural stem cell marker Nestin [69], the neuronal precursor marker CXCR4 [70] and KCa3.1 did not disclose any association of the growth phenotype with the abundance of Notch1 or Nestin (Fig. 1B). In contrast, ALDH1A3 and KCa3.1 showed a weak positive correlation (Pearson correlation coefficient PC = 0.44 and 0.48, respectively) while CXCR4 correlated moderately in a negative manner (PC = - 0.53) with the morphology score (Fig. 1B). This might hint to a more spheroid phenotype of CXCR4-positive proneural and a more adherent growth phenotype of mesenchymal glioblastoma stem cells and KCa3.1-expressing cells.
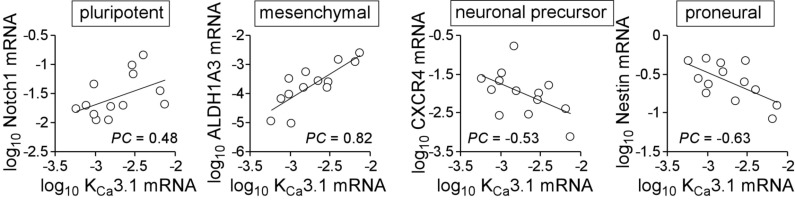
Next, we directly analyzed the KCa3.1 mRNA abundance in the primary glioblastoma cultures in dependence on stem cell marker expression. The data given in (Fig. 2) suggest a moderate negative correlation of KCa3.1 with CXCR4 (PC = -0.54) and Nestin (PC = -0.64) as well as a weak positive correlation with Notch1 (PC = 0.48) and a highly positive correlation with ALDH1A3 (PC = 0.82) hinting to an upregulation of KCa3.1 in mesenchymal stem cell-enriched glioblastoma.
Fig. (2).
Association of KCa3.1 mRNA abundance with stem cell markers in primary glioblastoma cultures (PC: Pearson correlation coefficient). Messenger RNAs were isolated and reversely transcribed as described [40]. Notch1-, ALDH1A3-, CXCR4-, Nestin-, KCNN4 (KCa3.1) and housekeeper β-actin (ACTB)-, pyruvate dehydrogenase beta (PDHB)-, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific fragments were amplified by the use of SYBR Green-based quantitative real-time PCR (QuantiTect Primer Assay QT01005109, QT00077588, QT00223188, QT00235781, QT00003780, QT01192646, QT00095431, and QT00031227, QIAGEN, Venlo, Netherlands, and 1Step RT qPCR Green ROX L Kit, highQu, Kraichtal, Germany) in a Roche LightCycler. Abundances of the individual mRNAs were normalized to the geometrical mean of the three housekeeper mRNAs.
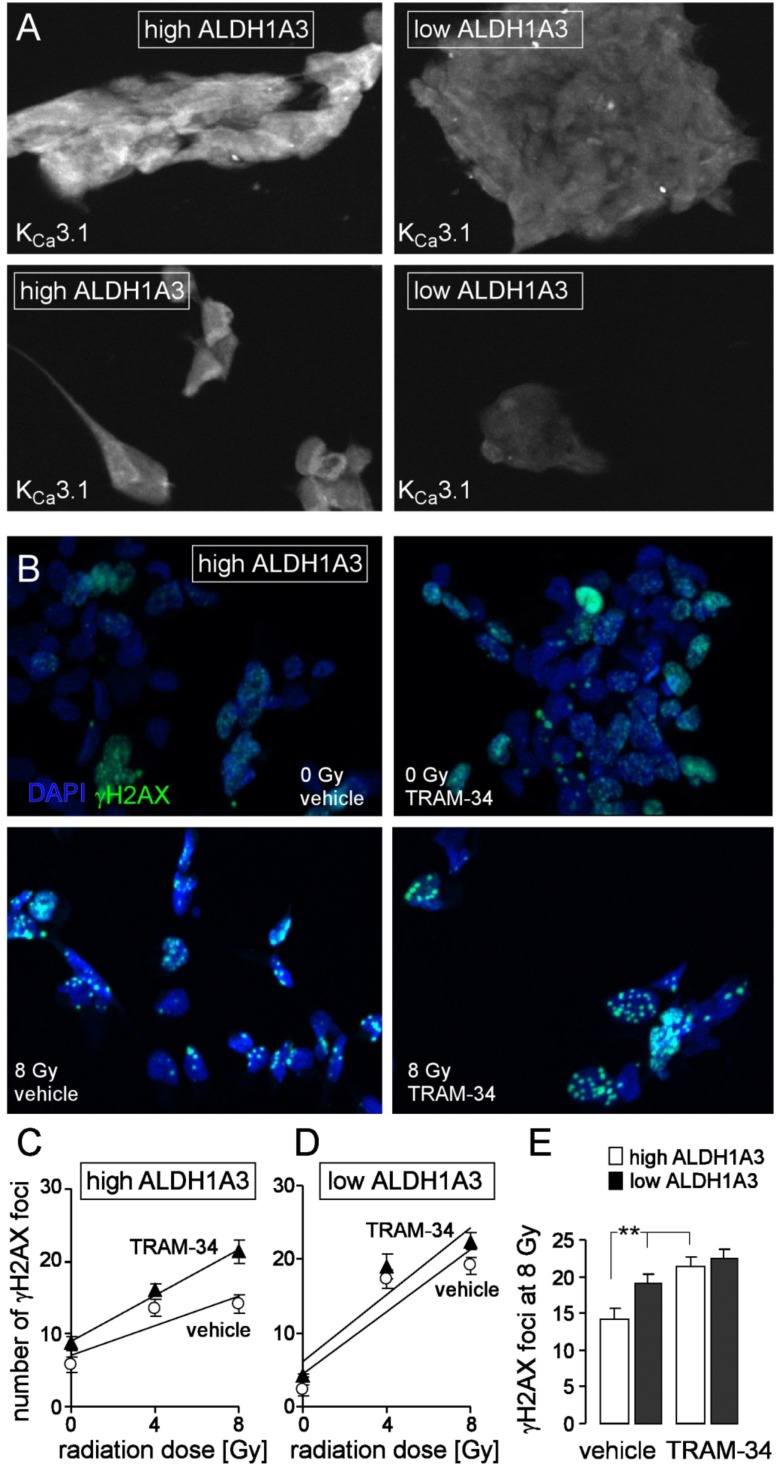
To determine the functional significance of KCa3.1 for the radioresistance of mesenchymal stem cell-enriched glioblastoma cultures, residual γH2AX foci were counted as a surrogate of unrepaired DNA double strand breaks 24 h after irradiation with 0, 4 or 8 Gy. A primary culture with high ALDH1A3 and KCa3.1 expression (Fig. 3A, left) and – for reference – a culture with low ALDH1A3 and KCa3.1 expression (Fig. 3A, right) were treated during irradiation and the following 24 h-incubation period with either vehicle alone or TRAM-34 (1 µM). Residual nuclear γH2AX foci were visualized by enhanced immunofluorescence microscopy (Fig. 3B) and foci number was plotted against the radiation dose (Fig. 3C and 3D). The slope of the foci number/radiation dose curve has been suggested to reflect the intrinsic radiosensitivity of the tumor cells [71] and was significantly steeper in cells with low as compared to those with high ALDH1A3 expression (compare open circles between Fig. 3C and 3D). Importantly, TRAM-34 radiosensitized only the culture with high ALDH1A3 expression (Fig. 3C, closed triangles) while having no effect in the low ALDH1A3-expressing cells (Fig. 3D, closed triangles). Accordingly, TRAM-34 significantly increased the number of residual γH2AX foci 24 h after irradiation with 8 Gy in the high ALDH1A3-expressing but not in the low ALDH1A3-expressing culture (Fig. 3E). Combined, these experiments confirm our previous data acquired in cell lines on the KCa3.1-mediated radioresistance in glioblastoma. Moreover, the present experiments suggest that KCa3.1-mediated radioresistance is largely confined to mesenchymal glioblastoma stem cells.
Fig. (3).
The KCa3.1 channel inhibitor TRAM-34 radiosensitizes mesenchymal stem cell-enriched glioblastoma cultures. A. Immunofluorescence micrographs of an ALDH1A3 high- (left) and an ALDH1A3 low-expressing (right) glioblastoma culture probed against KCa3.1 protein (white). B. Immunofluorescence staining of residual γH2AX foci (green) as measure of residual DNA double strand breaks in a high ALDH1A3-expressing glioblastoma culture (same culture as in A, left) 24 h after irradiation with 0 (upper line) or 8 Gy (lower line). Cells were irradiated and 24 h post-incubated in the presence of vehicle alone (left) or TRAM-34 (1 µM). Nuclei were counterstained with DAPI (blue). C, D. Mean (± SE, n = 42-100) number of residual γH2AX foci plotted against the radiation dose of high ALDH1A3- (C) and low ALDH1A3-expressing (D) glioblastoma cells 24 h after irradiation in the presence (closed triangles) or absence (open circles) of TRAM-34. E. Mean residual foci (data from C, D) 24 h after irradiation with 8 Gy in vehicle- (left) and TRAM-34-treated high (open bars) and low (closed bars) ALDH1A3-expressing glioblastoma (** indicates p ≤ 0.01, two-tailed t-test after Bonferroni correction for multiple comparisons). For immunofluorescence microscopy 1500 cells/well were seeded on a Millicell EZ SLIDE 8-well glass (Merck Millipore, Tullagreen, Ireland) and grown for 7 days, irradiated in the presence or absence (DMSO vehicle) of TRAM-34 (1 µM, Sigma-Aldrich, Deisenhofen, Germany) with 0, 4 or 8 Gy with 6 MV photons using a linear accelerator at a dose rate of 4 Gy/min, further incubated for 24 h with TRAM-34 and vehicle alone, respectively, and fixed with 3.7% formaldehyde for 15 min at 21°C followed by three washing steps with PBS. Next, cells were permeabilized for 10 min at 21°C with 0.1% Triton-X 100 in PBS followed again by three washing steps with PBS. Cells were probed against γH2AX and KCa3.1 protein using anti-γH2AX antibody (monoclonal mouse, Merck Millipore) and the anti-KCa3.1 antibody (polyclonal rabbit; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) in 1:1000 and 1:50 dilution, respectively, and antibody binding was visualized with the Tyramide Signal Amplification Kits for mouse and rabbit primary antibodies (Molecular Probes, Eugene, Oregon, USA). Prior to mounting, nuclei were counterstained with DAPI (Sigma-Aldrich). (The color version of the figure is available in the electronic copy of the article).
Concluding Remarks
The in vitro data on KCa3.1 Ca2+-activated K+ channels suggest multiple biological functions of these channels for tumor cells. In particular in glioblastoma, KCa3.1 has been identified as a key regulator of glioblastoma cell migration and DNA damage response suggesting that KCa3.1 promotes brain infiltration and intrinsic radioresistance in vivo. The original data set of the present article suggests an upregulation of KCa3.1 especially in the mesenchymal subtype of glioblastoma stem cells. In vivo, induction and maintenance of the highly malignant “stemness” phenotype have been proposed to depend on the reciprocal interaction of glioblastoma cells with endothelial cells in perivascular stem cell niches [72-74]. In this context, CD133+ glioblastoma stem cells have been shown to exhibit higher radioresistance than CD133- cells after xenografting into mouse brain pointing to an induction of radioresistance by the tumor microenvironment [75]. Chemotaxis [32, 36] including SDF1-induced chemotaxis [39] reportedly depends on KCa3.1. Moreover, formation of the perivascular stem cell niche requires SDF1/CXCR4 signaling [76]. Therefore, one might speculate that beyond conferring intrinsic radioresistance, KCa3.1 also contributes to SDF1/CXCR4-guided homing of glioblastoma cells to perivascular stem cell niches and to the acquisition/maintenance of the radioresistant glioblastoma stem cell phenotype.
Hence, KCa3.1 might be a promising pharmacological target to prevent the induction and/or maintenance and to attenuate the radioresistance of in particular mesenchymal glioblastoma stem cells as suggested by the present article. As pointed out in the INTRODUCTION section, KCa3.1 is druggable and patients especially with the mesenchymal molecular subtype of glioblastoma might benefit from KCa3.1 targeting in combination with fractionated radiotherapy. For such a therapy strategy, the immune-modulating action of systemic KCa3.1 targeting must be taken into account. As a matter of fact, KCa3.1 targeting has been demonstrated to induce a switch of glioblastoma infiltrating microglia/macrophages towards a pro-inflammatory, anti-tumor phenotype [77] which is thought to improve the therapy outcome.
Acknowledgements
Lukas Klumpp was supported by the ICEPHA program of the University of Tübingen and the Robert-Bosch-Gesellschaft für Medizinische Forschung, Stuttgart. We thank Heidrun Faltin for excellent technical assistance.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Sforna L., Megaro A., Pessia M., Franciolini F., Catacuzzeno L. Structure, gating and basic functions of the Ca2+-activated K channel of intermediate conductance. Curr. Neuropharmacol. 2018;16:609–618. doi: 10.2174/1570159X15666170830122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim. Biophys. Acta. 1958;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [http://dx.doi.org/10.1016/0006-3002(58)90124-0]. [PMID: 13618284]. [DOI] [PubMed] [Google Scholar]
- 3.Lang P.A., Kaiser S., Myssina S., Wieder T., Lang F., Huber S.M. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell Physiol. 2003;285(6):C1553–C1560. doi: 10.1152/ajpcell.00186.2003. [http://dx.doi.org/10.1152/ajpcell.00186.2003]. [PMID: 14600080]. [DOI] [PubMed] [Google Scholar]
- 4.Föller M., Bobbala D., Koka S., Boini K.M., Mahmud H., Kasinathan R.S., Shumilina E., Amann K., Beranek G., Sausbier U., Ruth P., Sausbier M., Lang F., Huber S.M. Functional significance of the intermediate conductance Ca2+-activated K+ channel for the short-term survival of injured erythrocytes. Pflugers Arch. 2010;460(6):1029–1044. doi: 10.1007/s00424-010-0878-1. [http://dx.doi.org/10.1007/s00424-010-0878-1]. [PMID: 20857305]. [DOI] [PubMed] [Google Scholar]
- 5.Brugnara C., de Franceschi L., Alper S.L. Inhibition of Ca2+-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Invest. 1993;92(1):520–526. doi: 10.1172/JCI116597. [http://dx.doi.org/10.1172/JCI116597]. [PMID: 8326017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown B.M., Pressley B., Wulff H. KCa3.1 Channel Modulators as Potential Therapeutic Compounds for Glioblastoma. Curr. Neuropharmacol. 2018;16:619–627. doi: 10.2174/1570159X15666170630164226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber S.M., Tschöp J., Braun G.S., Nagel W., Horster M.F. Bradykinin-stimulated Cl-secretion in T84 cells. Role of Ca2+-activated hSK4-like K+ channels. Pflugers Arch. 1999;438(1):53–60. doi: 10.1007/s004240050879. [http://dx.doi.org/10.1007/s004240050879]. [PMID: 10370087]. [DOI] [PubMed] [Google Scholar]
- 8.Wei A.D., Gutman G.A., Aldrich R., Chandy K.G., Grissmer S., Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol. Rev. 2005;57(4):463–472. doi: 10.1124/pr.57.4.9. [http://dx.doi.org/10. 1124/pr.57.4.9]. [PMID: 16382103]. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.J., Wallace B.K., Yuen N., Jenkins D.P., Wulff H., O’Donnell M.E. Blood-brain barrier KCa3.1 channels: evidence for a role in brain Na uptake and edema in ischemic stroke. Stroke. 2015;46(1):237–244. doi: 10.1161/STROKEAHA.114.007445. [http://dx.doi.org/10.1161/STROKEAHA. 114.007445]. [PMID: 25477223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stegen B., Klumpp L., Misovic M., Edalat L., Eckert M., Klumpp D., Ruth P., Huber S.M.K. +channel signaling in irradiated tumor cells. Eur. Biophys. J. 2016;45(7):585–598. doi: 10.1007/s00249-016-1136-z. [http://dx.doi.org/10.1007/s00249-016-1136-z]. [PMID: 27165704]. [DOI] [PubMed] [Google Scholar]
- 11.Gamper N., Fillon S., Feng Y., Friedrich B., Lang P.A., Henke G., Huber S.M., Kobayashi T., Cohen P., Lang F.K. + channel activation by all three isoforms of serum- and glucocorticoid-dependent protein kinase SGK. Pflugers Arch. 2002;445(1):60–66. doi: 10.1007/s00424-002-0873-2. [http://dx.doi.org/10.1007/s00424-002-0873-2]. [PMID: 12397388]. [DOI] [PubMed] [Google Scholar]
- 12.Gamper N., Fillon S., Huber S.M., Feng Y., Kobayashi T., Cohen P., Lang F. IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1. Pflugers Arch. 2002;443(4):625–634. doi: 10.1007/s00424-001-0741-5. [http://dx.doi.org/10.1007/s00424-001-0741-5]. [PMID: 11907830]. [DOI] [PubMed] [Google Scholar]
- 13.Tanneur V., Ilgaz D., Duranton C., Fillon S., Gamper N., Huber S.M., Lang F. Time-dependent regulation of capacitative Ca2+ entry by IGF-1 in human embryonic kidney cells. Pflugers Arch. 2002;445(1):74–79. doi: 10.1007/s00424-002-0859-0. [http://dx.doi.org/10.1007/s00424-002-0859-0]. [PMID: 12397390]. [DOI] [PubMed] [Google Scholar]
- 14.Ghanshani S., Wulff H., Miller M.J., Rohm H., Neben A., Gutman G.A., Cahalan M.D., Chandy K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [http://dx.doi.org/10.1074/jbc.M003941200]. [PMID: 10961988]. [DOI] [PubMed] [Google Scholar]
- 15.D’Alessandro G., Limatola C., Catalanoa M. Functional roles of the Ca2+-activated K+ channel, KCa3.1, in brain tumors. Curr. Neuropharmacol. 2018;16:637–644. doi: 10.2174/0929867324666170713103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Marchi U., Sassi N., Fioretti B., Catacuzzeno L., Cereghetti G.M., Szabò I., Zoratti M. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium. 2009;45(5):509–516. doi: 10.1016/j.ceca.2009.03.014. [http://dx.doi.org/10.1016/j.ceca.2009.03.014]. [PMID: 19406468]. [DOI] [PubMed] [Google Scholar]
- 17.Sassi N., De Marchi U., Fioretti B., Biasutto L., Gulbins E., Franciolini F., Szabò I., Zoratti M. An investigation of the occurrence and properties of the mitochondrial intermediate-conductance Ca2+-activated K+ channel mtKCa3.1. Biochim. Biophys. Acta. 2010;1797(6-7):1260–1267. doi: 10.1016/j.bbabio.2009.12.015. [http://dx.doi.org/10.1016/j.bbabio.2009. 12.015]. [PMID: 20036632]. [DOI] [PubMed] [Google Scholar]
- 18.Kovalenko I., Glasauer A., Schöckel L., Sauter D.R., Ehrmann A., Sohler F., Hägebarth A., Novak I., Christian S. Identification of KCa3.1 Channel as a Novel Regulator of Oxidative Phosphorylation in a Subset of Pancreatic Carcinoma Cell Lines. PLoS One. 2016;11(8):e0160658. doi: 10.1371/journal.pone.0160658. [http://dx.doi.org/10.1371/journal.pone. 0160658]. [PMID: 27494181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth B., Gibhardt C.S., Becker P., Gebhardt M., Knoop J., Fournier C., Moroni A., Thiel G. Low-dose photon irradiation alters cell differentiation via activation of hIK channels. Pflugers Arch. 2015;467(8):1835–1849. doi: 10.1007/s00424-014-1601-4. [http://dx.doi.org/10.1007/s00424-014-1601-4]. [PMID: 25277267]. [DOI] [PubMed] [Google Scholar]
- 20.Dittmann K., Mayer C., Rodemann H.P., Huber S.M. EGFR cooperates with glucose transporter SGLT1 to enable chromatin remodeling in response to ionizing radiation. Radiother. Oncol. 2013;107(2):247–251. doi: 10.1016/j.radonc.2013.03.016. [http://dx.doi.org/10.1016/j.radonc.2013. 03.016]. [PMID: 23602371]. [DOI] [PubMed] [Google Scholar]
- 21.Huber S.M., Misovic M., Mayer C., Rodemann H.P., Dittmann K. EGFR-mediated stimulation of sodium/glucose cotransport promotes survival of irradiated human A549 lung adenocarcinoma cells. Radiother. Oncol. 2012;103(3):373–379. doi: 10.1016/j.radonc.2012.03.008. [http://dx.doi.org/ 10.1016/j.radonc.2012.03.008]. [PMID: 22516777]. [DOI] [PubMed] [Google Scholar]
- 22.Quast S.A., Berger A., Buttstädt N., Friebel K., Schönherr R., Eberle J. General Sensitization of melanoma cells for TRAIL-induced apoptosis by the potassium channel inhibitor TRAM-34 depends on release of SMAC. PLoS One. 2012;7(6):e39290. doi: 10.1371/journal.pone.0039290. [http://dx.doi.org/10.1371/journal.pone.0039290]. [PMID: 22723988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leanza L., O’Reilly P., Doyle A., Venturini E., Zoratti M., Szegezdi E., Szabo I. Correlation between potassium channel expression and sensitivity to drug-induced cell death in tumor cell lines. Curr. Pharm. Des. 2014;20(2):189–200. doi: 10.2174/13816128113199990032. [http://dx.doi.org/ 10.2174/13816128113199990032]. [PMID: 23701546]. [DOI] [PubMed] [Google Scholar]
- 24.McFerrin M.B., Turner K.L., Cuddapah V.A., Sontheimer H. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am. J. Physiol. Cell Physiol. 2012;303(10):C1070–C1078. doi: 10.1152/ajpcell.00040.2012. [http://dx.doi.org/10.1152/ ajpcell.00040.2012]. [PMID: 22992678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steudel F.A., Mohr C.J., Stegen B., Nguyen H.Y. Barnert, A., Steinle, M., Beer-Hammer, S., Koch, P., Lo, W.-Y., Schroth, W., Hoppe, R., Brauch, H., Ruth, P., Huber, S.M., Lukowski, P. SK4 channels modulate Ca2+-signalling and cell cycle progression in murine breast cancer. Mol. Oncol. 2017;11:1172–1188. doi: 10.1002/1878-0261.12087. [http://dx. doi.org/10.1002/1878-0261.12087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biasiotta A., D’Arcangelo D., Passarelli F., Nicodemi E.M., Facchiano A. Ion channels expression and function are strongly modified in solid tumors and vascular malformations. J. Transl. Med. 2016;14(1):285. doi: 10.1186/s12967-016-1038-y. [http://dx.doi.org/10.1186/s12967-016-1038-y]. [PMID: 27716384]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bulk E., Ay A.S., Hammadi M., Ouadid-Ahidouch H., Schelhaas S., Hascher A., Rohde C., Thoennissen N.H., Wiewrodt R., Schmidt E., Marra A., Hillejan L., Jacobs A.H., Klein H.U., Dugas M., Berdel W.E., Müller-Tidow C., Schwab A. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int. J. Cancer. 2015;137(6):1306–1317. doi: 10.1002/ijc.29490. [http://dx. doi.org/10.1002/ijc.29490]. [PMID: 25704182]. [DOI] [PubMed] [Google Scholar]
- 28.Jäger H., Dreker T., Buck A., Giehl K., Gress T., Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol. Pharmacol. 2004;65(3):630–638. doi: 10.1124/mol.65.3.630. [http://dx.doi.org/10.1124/mol.65.3.630]. [PMID: 14978241]. [DOI] [PubMed] [Google Scholar]
- 29.Lallet-Daher H., Roudbaraki M., Bavencoffe A., Mariot P., Gackière F., Bidaux G., Urbain R., Gosset P., Delcourt P., Fleurisse L., Slomianny C., Dewailly E., Mauroy B., Bonnal J.L., Skryma R., Prevarskaya N. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009;28(15):1792–1806. doi: 10.1038/onc.2009.25. [http://dx.doi.org/10.1038/onc.2009.25]. [PMID: 19270724]. [DOI] [PubMed] [Google Scholar]
- 30.Parihar A.S., Coghlan M.J., Gopalakrishnan M., Shieh C.C. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur. J. Pharmacol. 2003;471(3):157–164. doi: 10.1016/s0014-2999(03)01825-9. [http://dx.doi.org/10.1016/ S0014-2999(03)01825-9]. [PMID: 12826234]. [DOI] [PubMed] [Google Scholar]
- 31.Klumpp D., Misovic M., Szteyn K., Shumilina E., Rudner J., Huber S.M. Targeting TRPM2 channels impairs radiation-induced cell cycle arrest and fosters cell death of T cell leukemia cells in a Bcl-2-dependent manner. Oxid. Med. Cell. Longev. 2016;2016:8026702. doi: 10.1155/2016/8026702. [http://dx.doi.org/10.1155/2016/8026702]. [PMID: 26839633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catacuzzeno L., Aiello F., Fioretti B., Sforna L., Castigli E., Ruggieri P., Tata A.M., Calogero A., Franciolini F. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell. Physiol. 2011;226(7):1926–1933. doi: 10.1002/jcp.22523. [http://dx. doi.org/10.1002/jcp.22523]. [PMID: 21506123]. [DOI] [PubMed] [Google Scholar]
- 33.Weaver A.K., Bomben V.C., Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia. 2006;54(3):223–233. doi: 10.1002/glia.20364. [http://dx.doi.org/10.1002/ glia.20364]. [PMID: 16817201]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner K.L., Honasoge A., Robert S.M., McFerrin M.M., Sontheimer H. A proinvasive role for the Ca2+ -activated K+ channel KCa3.1 in malignant glioma. Glia. 2014;62(6):971–981. doi: 10.1002/glia.22655. [http://dx.doi.org/10.1002/glia.22655]. [PMID: 24585442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Alessandro G., Catalano M., Sciaccaluga M., Chece G., Cipriani R., Rosito M., Grimaldi A., Lauro C., Cantore G., Santoro A., Fioretti B., Franciolini F., Wulff H., Limatola C.K. Ca3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013;4:e773. doi: 10.1038/cddis.2013.279. [http://dx.doi.org/10.1038/ cddis.2013.279]. [PMID: 23949222]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuddapah V.A., Turner K.L., Seifert S., Sontheimer H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J. Neurosci. 2013;33(4):1427–1440. doi: 10.1523/JNEUROSCI.3980-12.2013. [http:// dx.doi.org/10.1523/JNEUROSCI.3980-12.2013]. [PMID: 23345219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Alessandro G., Grimaldi A., Chece G., Porzia A., Esposito V., Santoro A., Salvati M., Mainiero F., Ragozzino D., Di Angelantonio S., Wulff H., Catalano M., Limatola C.K. Ca3.1 channel inhibition sensitizes malignant gliomas to temozolomide treatment. Oncotarget. 2016;7(21):30781–30796. doi: 10.18632/oncotarget.8761. [http://dx.doi.org/10. 18632/oncotarget.8761]. [PMID: 27096953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stegen B., Butz L., Klumpp L., Zips D., Dittmann K., Ruth P., Huber S.M. Ca2+-Activated IK K+ Channel Blockade Radiosensitizes Glioblastoma Cells. Mol. Cancer Res. 2015;13(9):1283–1295. doi: 10.1158/1541-7786.MCR-15-0075. [http://dx.doi.org/10.1158/1541-7786.MCR-15-0075]. [PMID: 26041939]. [DOI] [PubMed] [Google Scholar]
- 39.Sciaccaluga M., Fioretti B., Catacuzzeno L., Pagani F., Bertollini C., Rosito M., Catalano M., D’Alessandro G., Santoro A., Cantore G., Ragozzino D., Castigli E., Franciolini F., Limatola C. CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am. J. Physiol. Cell Physiol. 2010;299(1):C175–C184. doi: 10.1152/ajpcell.00344.2009. [http://dx.doi.org/ 10.1152/ajpcell.00344.2009]. [PMID: 20392929]. [DOI] [PubMed] [Google Scholar]
- 40.Edalat L., Stegen B., Klumpp L., Haehl E., Schilbach K., Lukowski R., Kühnle M., Bernhardt G., Buschauer A., Zips D., Ruth P., Huber S.M.B.K.K. + channel blockade inhibits radiation-induced migration/brain infiltration of glioblastoma cells. Oncotarget. 2016;7(12):14259–14278. doi: 10.18632/oncotarget.7423. [http://dx.doi.org/10.18632/ oncotarget.7423]. [PMID: 26893360]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M.W., Li C.Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;26(1):63–74. doi: 10.1016/j.molcel.2007.02.024. [http://dx.doi.org/10.1016/j.molcel.2007.02.024]. [PMID: 17434127]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dittmann K., Mayer C., Kehlbach R., Rothmund M.C., Peter Rodemann H. Radiation-induced lipid peroxidation activates src kinase and triggers nuclear EGFR transport. Radiother. Oncol. 2009;92(3):379–382. doi: 10.1016/j.radonc.2009.06.003. [http://dx.doi.org/10.1016/j.radonc.2009. 06.003]. [PMID: 19560222]. [DOI] [PubMed] [Google Scholar]
- 43.Dittmann K., Mayer C., Paasch A., Huber S., Fehrenbacher B., Schaller M., Rodemann H.P. Nuclear EGFR renders cells radio-resistant by binding mRNA species and triggering a metabolic switch to increase lactate production. Radiother. Oncol. 2015;116(3):431–437. doi: 10.1016/j.radonc.2015.08.016. [http://dx.doi.org/10.1016/j.radonc.2015.08.016]. [PMID: 26320552]. [DOI] [PubMed] [Google Scholar]
- 44.Catacuzzeno L., Fioretti B., Franciolini F. A theoretical study on the role of Ca(2+)-activated K+ channels in the regulation of hormone-induced Ca2+ oscillations and their synchronization in adjacent cells. J. Theor. Biol. 2012;309:103–112. doi: 10.1016/j.jtbi.2012.05.009. [http://dx.doi.org/ 10.1016/j.jtbi.2012.05.009]. [PMID: 22659037]. [DOI] [PubMed] [Google Scholar]
- 45.Takuwa N., Zhou W., Takuwa Y. Calcium, calmodulin and cell cycle progression. Cell. Signal. 1995;7(2):93–104. doi: 10.1016/0898-6568(94)00074-l. [http://dx. doi.org/10.1016/0898-6568(94)00074-L]. [PMID: 7794690]. [DOI] [PubMed] [Google Scholar]
- 46.Steinle M., Palme D., Misovic M., Rudner J., Dittmann K., Lukowski R., Ruth P., Huber S.M. Ionizing radiation induces migration of glioblastoma cells by activating BK K(+) channels. Radiother. Oncol. 2011;101(1):122–126. doi: 10.1016/j.radonc.2011.05.069. [http://dx.doi.org/10.1016/ j.radonc.2011.05.069]. [PMID: 21704404]. [DOI] [PubMed] [Google Scholar]
- 47.Heise N., Palme D., Misovic M., Koka S., Rudner J., Lang F., Salih H.R., Huber S.M., Henke G. Non-selective cation channel-mediated Ca2+-entry and activation of Ca2+/calmodulin-dependent kinase II contribute to G2/M cell cycle arrest and survival of irradiated leukemia cells. Cell. Physiol. Biochem. 2010;26(4-5):597–608. doi: 10.1159/000322327. [http://dx.doi.org/10.1159/000322327]. [PMID: 21063097]. [DOI] [PubMed] [Google Scholar]
- 48.Palme D., Misovic M., Schmid E., Klumpp D., Salih H.R., Rudner J., Huber S.M. Kv3.4 potassium channel-mediated electrosignaling controls cell cycle and survival of irradiated leukemia cells. Pflugers Arch. 2013;465(8):1209–1221. doi: 10.1007/s00424-013-1249-5. [http://dx.doi.org/ 10.1007/s00424-013-1249-5]. [PMID: 23443853]. [DOI] [PubMed] [Google Scholar]
- 49.Ruggieri P., Mangino G., Fioretti B., Catacuzzeno L., Puca R., Ponti D., Miscusi M., Franciolini F., Ragona G., Calogero A. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS One. 2012;7(10):e47825. doi: 10.1371/journal.pone.0047825. [http://dx.doi.org/10.1371/journal.pone.0047825]. [PMID: 23110108]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwab A., Fabian A., Hanley P.J., Stock C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012;92(4):1865–1913. doi: 10.1152/physrev.00018.2011. [http://dx.doi.org/10.1152/physrev.00018.2011]. [PMID: 23073633]. [DOI] [PubMed] [Google Scholar]
- 51.Watkins S., Sontheimer H. Hydrodynamic cellular volume changes enable glioma cell invasion. J. Neurosci. 2011;31(47):17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.3938-11. 2011]. [PMID: 22114291]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu G., Yuan X., Zeng Z., Tunici P., Ng H., Abdulkadir I.R., Lu L., Irvin D., Black K.L., Yu J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [http://dx.doi.org/10.1186/1476-4598-5-67]. [PMID: 17140455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [http://dx.doi.org/10.1038/nature05236]. [PMID: 17051156]. [DOI] [PubMed] [Google Scholar]
- 54.Joo K.M., Jin J., Kim E., Ho Kim K., Kim Y., Kang G. B.; Kang, Y.J.; Lathia, J.D.; Cheong, K.H.; Song, P.H.; Kim, H.; Seol, H.J.; Kong, D.S.; Lee, J.I.; Rich, J.N.; Lee, J.; Nam, D.H. MET signaling regulates glioblastoma stem cells. Cancer Res. 2012;72(15):3828–3838. doi: 10.1158/0008-5472.CAN-11-3760. [http://dx.doi.org/10.1158/0008-5472.CAN-11-3760]. [PMID: 22617325]. [DOI] [PubMed] [Google Scholar]
- 55.Nakada M., Nambu E., Furuyama N., Yoshida Y., Takino T., Hayashi Y., Sato H., Sai Y., Tsuji T., Miyamoto K.I., Hirao A., Hamada J.I. Integrin α3 is overexpressed in glioma stem-like cells and promotes invasion. Br. J. Cancer. 2013;108(12):2516–2524. doi: 10.1038/bjc.2013.218. [http://dx.doi.org/10.1038/bjc.2013.218]. [PMID: 23652300]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G.R., Deng X.L., Sun H., Chung S.S., Tse H.F., Lau C.P. Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells. 2006;24(6):1519–1528. doi: 10.1634/stemcells.2005-0307. [http://dx.doi.org/10.1634/ stemcells.2005-0307]. [PMID: 16484345]. [DOI] [PubMed] [Google Scholar]
- 57.Wang S.P., Wang J.A., Luo R.H., Cui W.Y., Wang H. Potassium channel currents in rat mesenchymal stem cells and their possible roles in cell proliferation. Clin. Exp. Pharmacol. Physiol. 2008;35(9):1077–1084. doi: 10.1111/j.1440-1681.2008.04964.x. [http://dx.doi.org/10.1111/j.1440-1681. 2008.04964.x]. [PMID: 18505444]. [DOI] [PubMed] [Google Scholar]
- 58.Turner K.L., Sontheimer H.K. Ca3.1 modulates neuroblast migration along the rostral migratory stream (RMS) in vivo. Cereb. Cortex. 2014;24(9):2388–2400. doi: 10.1093/cercor/bht090. [http://dx.doi.org/10.1093/cercor/ bht090]. [PMID: 23585521]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., Alexe G., Lawrence M., O’Kelly M., Tamayo P., Weir B.A., Gabriel S., Winckler W., Gupta S., Jakkula L., Feiler H.S., Hodgson J.G., James C.D., Sarkaria J.N., Brennan C., Kahn A., Spellman P.T., Wilson R.K., Speed T.P., Gray J.W., Meyerson M., Getz G., Perou C.M., Hayes D.N. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [http://dx.doi.org/10.1016/j.ccr.2009. 12.020]. [PMID: 20129251]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao P., Joshi K., Li J., Kim S.H., Li P., Santana-Santos L., Luthra S., Chandran U.R., Benos P.V., Smith L., Wang M., Hu B., Cheng S.Y., Sobol R.W., Nakano I. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc. Natl. Acad. Sci. USA. 2013;110(21):8644–8649. doi: 10.1073/pnas.1221478110. [http://dx.doi.org/10.1073/pnas. 1221478110]. [PMID: 23650391]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandran U.R., Luthra S., Santana-Santos L., Mao P., Kim S.H., Minata M., Li J., Benos P.V., DeWang M., Hu B., Cheng S.Y., Nakano I., Sobol R.W. Gene expression profiling distinguishes proneural glioma stem cells from mesenchymal glioma stem cells. Genom. Data. 2015;5:333–336. doi: 10.1016/j.gdata.2015.07.007. [http://dx.doi.org/ 10.1016/j.gdata.2015.07.007]. [PMID: 26251826]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balbous A., Cortes U., Guilloteau K., Villalva C., Flamant S., Gaillard A., Milin S., Wager M., Sorel N., Guilhot J., Bennaceur-Griscelli A., Turhan A., Chomel J.C., Karayan-Tapon L. A mesenchymal glioma stem cell profile is related to clinical outcome. Oncogenesis. 2014;3:e91. doi: 10.1038/oncsis.2014.5. [http://dx.doi.org/10.1038/ oncsis.2014.5]. [PMID: 24637491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcato P., Dean C.A., Pan D., Araslanova R., Gillis M., Joshi M., Helyer L., Pan L., Leidal A., Gujar S., Giacomantonio C.A., Lee P.W. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29(1):32–45. doi: 10.1002/stem.563. [http://dx. doi.org/10.1002/stem.563]. [PMID: 21280157]. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W., Liu Y., Hu H., Huang H., Bao Z., Yang P., Wang Y., You G., Yan W., Jiang T., Wang J., Zhang W. ALDH1A3: A Marker of Mesenchymal Phenotype in Gliomas Associated with Cell Invasion. PLoS One. 2015;10(11):e0142856. doi: 10.1371/journal.pone.0142856. [http://dx. doi.org/10.1371/journal.pone.0142856]. [PMID: 26575197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flahaut M., Jauquier N., Chevalier N., Nardou K., Balmas Bourloud K., Joseph J.M., Barras D., Widmann C., Gross N., Renella R., Mühlethaler-Mottet A. Aldehyde dehydrogenase activity plays a Key role in the aggressive phenotype of neuroblastoma. BMC Cancer. 2016;16(1):781. doi: 10.1186/s12885-016-2820-1. [http://dx.doi.org/10.1186/s12885-016-2820-1]. [PMID: 27724856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurth I., Hein L., Mäbert K., Peitzsch C., Koi L., Cojoc M., Kunz-Schughart L., Baumann M., Dubrovska A. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget. 2015;6(33):34494–34509. doi: 10.18632/oncotarget.5417. [http://dx. doi.org/10.18632/oncotarget.5417]. [PMID: 26460734]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canino C., Luo Y., Marcato P., Blandino G., Pass H.I., Cioce M.A. STAT3-NFkB/DDIT3/CEBPβ axis modulates ALDH1A3 expression in chemoresistant cell subpopulations. Oncotarget. 2015;6(14):12637–12653. doi: 10.18632/oncotarget.3703. [http://dx.doi.org/10.18632/oncotarget. 3703]. [PMID: 25868979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noisa P., Lund C., Kanduri K., Lund R., Lähdesmäki H., Lahesmaa R., Lundin K., Chokechuwattanalert H., Otonkoski T., Tuuri T., Raivio T. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J. Cell Sci. 2014;127(Pt 9):2083–2094. doi: 10.1242/jcs.145755. [http://dx.doi.org/10.1242/jcs.145755]. [PMID: 24569875]. [DOI] [PubMed] [Google Scholar]
- 69.Lendahl U., Zimmerman L.B., McKay R.D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [http://dx.doi.org/10.1016/0092-8674(90)90662-X]. [PMID: 1689217]. [DOI] [PubMed] [Google Scholar]
- 70.Peng H., Kolb R., Kennedy J.E., Zheng J. Differential expression of CXCL12 and CXCR4 during human fetal neural progenitor cell differentiation. J. Neuroimmune Pharmacol. 2007;2(3):251–258. doi: 10.1007/s11481-007-9081-3. [http://dx.doi.org/10.1007/s11481-007-9081-3]. [PMID: 18040858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menegakis A., Eicheler W., Yaromina A., Thames H.D., Krause M., Baumann M. Residual DNA double strand breaks in perfused but not in unperfused areas determine different radiosensitivity of tumours. Radiother. Oncol. 2011;100(1):137–144. doi: 10.1016/j.radonc.2011.07.001. [http://dx.doi. org/10.1016/j.radonc.2011.07.001]. [PMID: 21821302]. [DOI] [PubMed] [Google Scholar]
- 72.Anido J., Sáez-Borderías A., Gonzàlez-Juncà A., Rodón L., Folch G., Carmona M.A., Prieto-Sánchez R.M., Barba I., Martínez-Sáez E., Prudkin L., Cuartas I., Raventós C., Martínez-Ricarte F., Poca M.A., García-Dorado D., Lahn M.M., Yingling J.M., Rodón J., Sahuquillo J., Baselga J., Seoane J. TGF-β receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18(6):655–668. doi: 10.1016/j.ccr.2010.10.023. [http://dx.doi.org/10.1016/j.ccr.2010.10.023]. [PMID: 21156287]. [DOI] [PubMed] [Google Scholar]
- 73.Rao S., Sengupta R., Choe E.J., Woerner B.M., Jackson E., Sun T., Leonard J., Piwnica-Worms D., Rubin J.B. CXCL12 mediates trophic interactions between endothelial and tumor cells in glioblastoma. PLoS One. 2012;7(3):e33005. doi: 10.1371/journal.pone.0033005. [http://dx. doi.org/10.1371/journal.pone.0033005]. [PMID: 22427929]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pietras A., Katz A.M., Ekström E.J., Wee B., Halliday J.J., Pitter K.L., Werbeck J.L., Amankulor N.M., Huse J.T., Holland E.C. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–369. doi: 10.1016/j.stem.2014.01.005. [http://dx. doi.org/10.1016/j.stem.2014.01.005]. [PMID: 24607407]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jamal M., Rath B.H., Tsang P.S., Camphausen K., Tofilon P.J. The brain microenvironment preferentially enhances the radioresistance of CD133(+) glioblastoma stem-like cells. Neoplasia. 2012;14(2):150–158. doi: 10.1593/neo.111794. [http://dx.doi.org/10.1593/neo.111794]. [PMID: 22431923]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson P.J. CXCR4 and glioblastoma. Anticancer. Agents Med. Chem. 2016;16(1):59–74. doi: 10.2174/1871520615666150824153032. [http://dx.doi.org/10.2174/ 1871520615666150824153032]. [PMID: 26299663]. [DOI] [PubMed] [Google Scholar]
- 77.Grimaldi A., D’Alessandro G., Golia M.T., Grössinger E.M., Di Angelantonio S., Ragozzino D., Santoro A., Esposito V., Wulff H., Catalano M., Limatola C.K. Ca3.1 inhibition switches the phenotype of glioma-infiltrating microglia/macrophages. Cell Death Dis. 2016;7:e2174. doi: 10.1038/cddis.2016.73. [http://dx.doi.org/10.1038/cddis.2016.73]. [PMID: 27054329]. [DOI] [PMC free article] [PubMed] [Google Scholar]