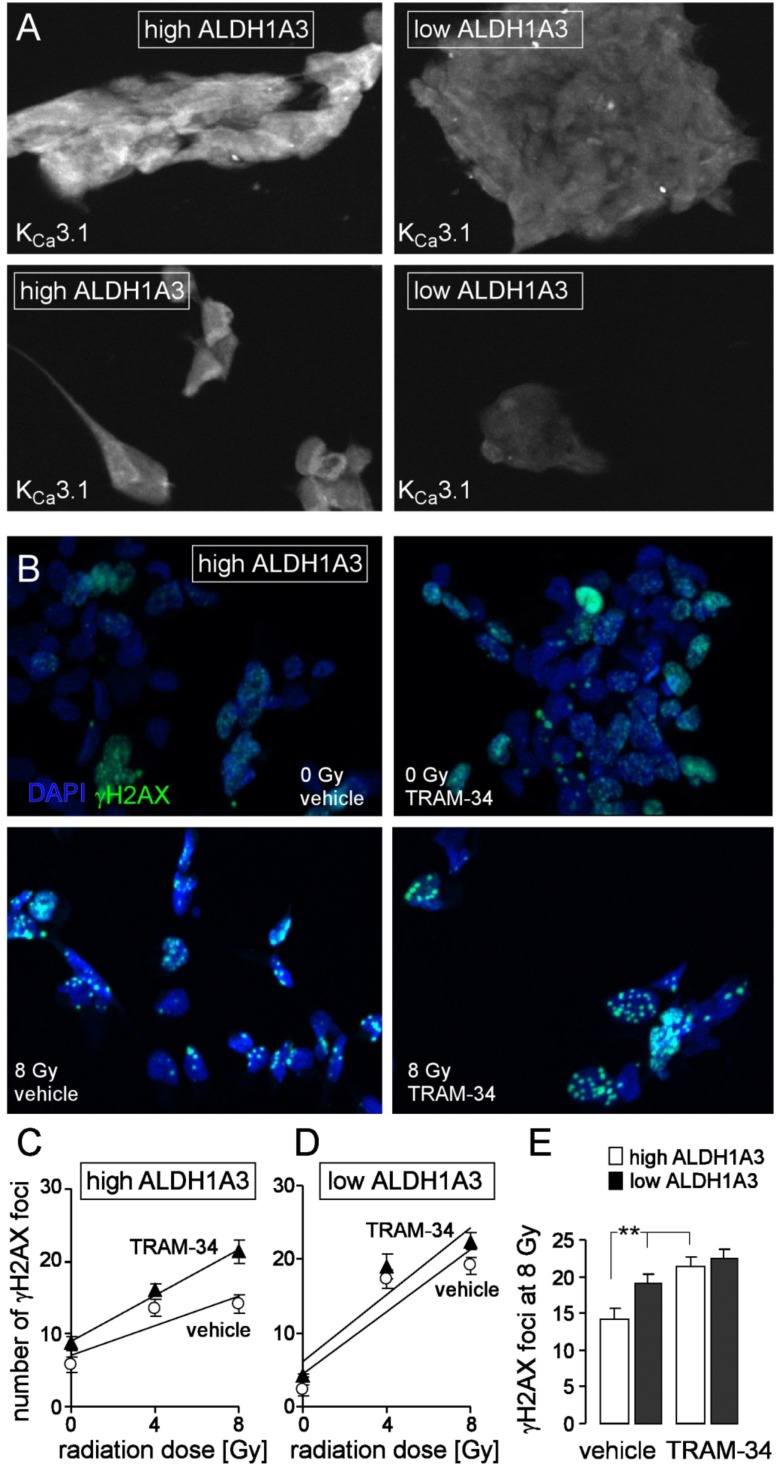
Fig. (3).
The KCa3.1 channel inhibitor TRAM-34 radiosensitizes mesenchymal stem cell-enriched glioblastoma cultures. A. Immunofluorescence micrographs of an ALDH1A3 high- (left) and an ALDH1A3 low-expressing (right) glioblastoma culture probed against KCa3.1 protein (white). B. Immunofluorescence staining of residual γH2AX foci (green) as measure of residual DNA double strand breaks in a high ALDH1A3-expressing glioblastoma culture (same culture as in A, left) 24 h after irradiation with 0 (upper line) or 8 Gy (lower line). Cells were irradiated and 24 h post-incubated in the presence of vehicle alone (left) or TRAM-34 (1 µM). Nuclei were counterstained with DAPI (blue). C, D. Mean (± SE, n = 42-100) number of residual γH2AX foci plotted against the radiation dose of high ALDH1A3- (C) and low ALDH1A3-expressing (D) glioblastoma cells 24 h after irradiation in the presence (closed triangles) or absence (open circles) of TRAM-34. E. Mean residual foci (data from C, D) 24 h after irradiation with 8 Gy in vehicle- (left) and TRAM-34-treated high (open bars) and low (closed bars) ALDH1A3-expressing glioblastoma (** indicates p ≤ 0.01, two-tailed t-test after Bonferroni correction for multiple comparisons). For immunofluorescence microscopy 1500 cells/well were seeded on a Millicell EZ SLIDE 8-well glass (Merck Millipore, Tullagreen, Ireland) and grown for 7 days, irradiated in the presence or absence (DMSO vehicle) of TRAM-34 (1 µM, Sigma-Aldrich, Deisenhofen, Germany) with 0, 4 or 8 Gy with 6 MV photons using a linear accelerator at a dose rate of 4 Gy/min, further incubated for 24 h with TRAM-34 and vehicle alone, respectively, and fixed with 3.7% formaldehyde for 15 min at 21°C followed by three washing steps with PBS. Next, cells were permeabilized for 10 min at 21°C with 0.1% Triton-X 100 in PBS followed again by three washing steps with PBS. Cells were probed against γH2AX and KCa3.1 protein using anti-γH2AX antibody (monoclonal mouse, Merck Millipore) and the anti-KCa3.1 antibody (polyclonal rabbit; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) in 1:1000 and 1:50 dilution, respectively, and antibody binding was visualized with the Tyramide Signal Amplification Kits for mouse and rabbit primary antibodies (Molecular Probes, Eugene, Oregon, USA). Prior to mounting, nuclei were counterstained with DAPI (Sigma-Aldrich). (The color version of the figure is available in the electronic copy of the article).