Abstract
Background
The intermediate-conductance Ca2+-activated K+ channel KCa3.1 is widely expressed in cells of the immune system such as T- and B-lymphocytes, mast cells, macrophages and microglia, but also found in dedifferentiated vascular smooth muscle cells, fibroblasts and many cancer cells including pancreatic, prostate, leukemia and glioblastoma. In all these cell types KCa3.1 plays an important role in cellular activation, migration and proliferation by regulating membrane potential and Ca2+ signaling.
Methods and Results
KCa3.1 therefore constitutes an attractive therapeutic target for diseases involving excessive proliferation or activation of one more of these cell types and researchers both in academia and in the pharmaceutical industry have developed several potent and selective small molecule inhibitors of KCa3.1. This article will briefly review the available compounds (TRAM-34, senicapoc, NS6180), their binding sites and mechanisms of action, and then discuss the potential usefulness of these compounds for the treatment of brain tumors based on their brain penetration and their efficacy in reducing microglia activation in animal models of ischemic stroke and Alzheimer’s disease.
Conclusion
Senicapoc, which has previously been in Phase III clinical trials, would be available for repurposing, and could be used to quickly translate findings made with other KCa3.1 blocking tool compounds into clinical trials.
Keywords: KCa3.1, TRAM-34, senicapoc, NS6180, repurposing, neuroinflammation, glioblastoma
1. INTRODUCTION
Glioblastoma multiforme (GBM) is the most common and aggressive form of brain tumors [1, 2]. They are highly invasive and migratory which make complete surgical removal nearly impossible. Median patient survival time is currently 15 months [1] due to the resistance of glioblastomas to conventional cancer therapies; there therefore exists great interest in identifying novel treatments or at least methods that could keep glioblastoma cells stationary to improve current palliative therapies (surgery, radiotherapy, chemotherapy).
The intermediate-conductance Ca2+-activated K+ channel KCa3.1 is a voltage-independent K+ channel that opens when increases in intracellular calcium activate the channel associated calmodulin at the intracellular C-terminal calmodulin binding domain [3]. KCa3.1 was first identified in red blood cells, where the channel is responsible for the so called Gárdos phenomenon, a Ca2+-activated K+ efflux [4, 5], and plays an important role in volume regulation [6]. Channel activation leads to K+ efflux, cellular dehydration and ultimately a reduction in cell volume. In addition to erythrocytes, KCa3.1 is widely expressed in peripheral tissues [7] such as vascular endothelium [8], secretory epithelia of the lung and gastrointestinal tract [9], as well as immune cells including T- and B-lymphocytes [10], mast cells [11], and macrophages [12]. KCa3.1 channels are further found in proliferating cells such as dedifferentiated vascular smooth muscle [13] and fibroblasts [14], as well as various cancer cell types, including pancreatic [15], prostate [16], and leukemia [17]. In immune cells, KCa3.1 provides the hyperpolarization necessary for facilitating Ca2+ entry through Ca2+-release activated Ca2+ (CRAC) channels during Ca2+ signaling following for example T cell or B cell receptor engagement [18, 19], while in smooth muscle cells and fibroblasts the channel “drives” proliferation subsequent to activation by PDGF, FGF or TGFβ [14]. In keeping with this expression pattern and the channel’s physiological functions, KCa3.1-/- mice exhibit impaired volume regulation in erythrocytes and T cells [6], impaired endothelial hyperpolarization with a small increase in mean arterial blood pressure [8] and blunted mast cell activation [11] but are otherwise healthy, have a normal life expectancy and reproduce normally.
In addition to its wide expression in the above mentioned peripheral tissues, KCa3.1 is also expressed in the brain, where the channel is primarily localized in microglia and has been shown to be involved in respiratory bursting, migration, proliferation, and lipopolysaccharide or amyloid-β oligomer induced nitric oxide production [20-22], as well as in microglia-mediated neuronal killing in cultures and organotypic hippocampal slices [21, 23]. In contrast to microglia, KCa3.1 expression in CNS neurons has always been somewhat controversial. After the Turner group published in 2015 that KCa3.1 is responsible for the slow afterhyperpolarization in hippocampal CA1 and CA3a neurons [24], John Adelman’s group demonstrated in early 2016 that KCa3.1 is not the sAHP since the current does not have the right pharmacological properties and is still present in KCa3.1-/- mice [25].
As discussed in greater detail in the accompanying articles in this thematic issue of Current Neuropharmacology, KCa3.1 has been proposed as a target for the treatment of glioblastomas because of its expression in glioblastoma cells themselves [26] and tumor associated microglia and macrophages [27, 28]. In the following sections, we will first briefly review the history of the small molecule KCa3.1 inhibitors TRAM-34, senicapoc and NS6180, including their binding sites and mechanisms of action, and then discuss the potential usefulness of these compounds for the treatment of brain tumors based on their brain penetration and available animal model and clinical experience with these compounds. Peptidic KCa3.1 blockers like the scorpion toxins charybdotoxin, its derivative ChTX-Glu32 [29] and maurotoxin [30, 31] will not be discussed here, since none of them have been used in vivo to inhibit KCa3.1.
1.1. TRAM-34
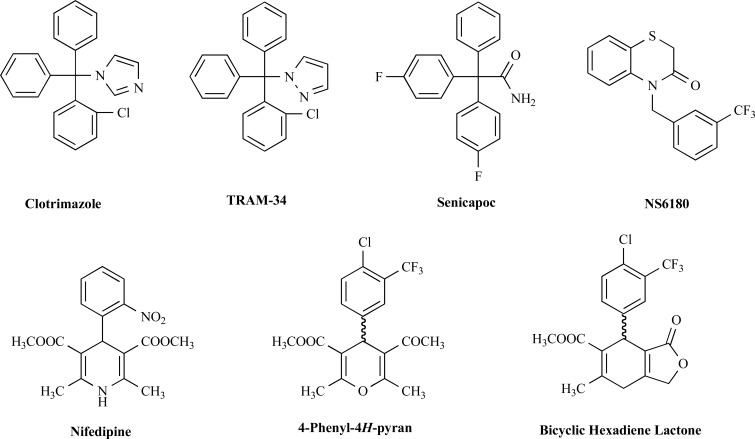
Our group designed TRAM-34 in 2000 as a derivative of the azole antimycotic clotrimazole [32]. While clotrimazole (Fig. 1) was the first identified nanomolar inhibitor of KCa3.1 [33], it also potently blocks cytochrome P450 enzymes leading to liver damage [34, 35], limiting any potential long-term therapeutic applications such as treatment of cancer and sickle cell anemia as were discussed for clotrimazole in the late 1990s [36, 37]. Nevertheless, clotrimazole was used as a KCa3.1 inhibitor in a small investigator initiated clinical trial in 5 patients with sickle cell anemia and found to reduce erythrocyte dehydration and increase erythrocyte K+ content [38]. However, as expected, the patients showed a concomitant increase in plasma liver enzymes [38]. When designing TRAM-34 (Fig. 1), we therefore preserved the triarylmethane structure present in clotrimazole but replaced the toxicity causing imidazole ring (the free electron pair on the imidazole complexes the iron in the heme moiety of P450 enzymes) with a pyrazole ring [32]. In comparison to clotrimazole (IC50 70-250 nM), TRAM-34 is more potent on KCa3.1 with an IC50 value of 20 nM and more selective (200–1000-fold) over other related KCa or more distantly related KV, NaV and TRP channels [32, 39]. These selectivity screens included ion channels associated with cardiac and smooth muscle cell function such as hERG (KV11.1), KCa1.1 (BK), KV1.5, CaV1.2 and TRPV4 [32, 39].
Fig. (1).
Chemical structures of KCa3.1 inhibitors.
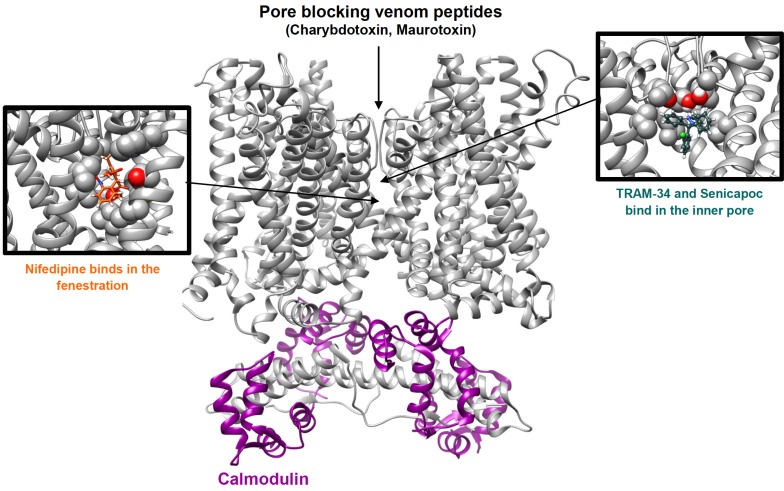
TRAM-34 blocks KCa3.1 currents by binding in the inner pore of the channel, interacting with threonine 250 in the pore loop and valine 275 in S6 as demonstrated by the fact that mutations of T250 and/or V275 completely abolish the sensitivity of KCa3.1 to inhibition by TRAM-34 and other triarylmethanes [40]. Based on a recent study using the Rosetta molecular modeling suite [41], TRAM-34 is situated near the threonine side chains from all four subunits of the tetrameric channel and blocks ion conduction by occupying the site that would normally be occupied by a K+ ion before it enters the selectivity filter. The pyrazole ring of TRAM-34 is positioned between the valine 275 side chains from adjacent subunits with the pyrazole nitrogen forming a hydrogen bond with the T250 side-chain from one of the subunits (Fig. 2).
Fig. (2).
Cartoon illustrating the binding sites of the triarylmethanes TRAM-34 and senicapoc in the inner pore and nifedipine in the fenestration region of KCa3.1. The tetramer of KCa3.1 alpha-subunits is shown as a grey ribbon. The channel associated calmodulin is shown in dark magenta. (The color version of the figure is available in the electronic copy of the article).
Based on its selectivity and its acceptable pharmacokinetic properties when administered intraperitoneally or subcutaneously [42, 43], TRAM-34 is a useful pharmacological tool compound to study the role of KCa3.1 in physiological and pathophysiological processes. For example, TRAM-34 has been used for proof-of-concept animal studies validating KCa3.1 as a potential therapeutic target for vascular restenosis [13, 44], atherosclerosis [43], asthma [45], allograft vasculopathy [46], obliterative airway disease [47], inflammatory bowel disease [48], and renal [14, 49] and cardiac fibrosis [50, 51]. In all these disease models, many of which were confirmed by parallel experiments in KCa3.1-/- mice, KCa3.1 expression was seen on multiple cell types involved in disease pathology such as proliferating vascular smooth muscles and fibroblasts as well as infiltrating T cells and macrophages. Of more relevance to the application of KCa3.1 blockers to glioblastoma treatment, TRAM-34, which is able to cross the blood brain barrier and has a brain/plasma ratio of 1.2/1 [42], has also been used to inhibit microglia activation and has been shown by our group to reduce infarction and improve neurological deficit in both a rat and a mouse model of ischemia/reperfusion stroke when administration is started 12 hours after reperfusion [23, 42]. TRAM-34 has also been found to reduce microglia activation induced by hippocampal injection of oligomeric amyloid-β in rats and to improve performance in a hippocampus-dependent novel object recognition task in mice with induced amyloid-β accumulation [52].
1.2. Senicapoc
In 2003, scientists at Icagen Inc. in North Carolina first published another compound based on the structure of clotrimazole [53]. Originally known as ICA-17043, senicapoc, like TRAM-34, improved in terms of both potency and selectivity for KCa3.1, blocking KCa3.1 with an IC50 of 11 nM [53, 54]. Senicapoc is a fluorinated triphenyl acetamide (Fig. 1) that very likely blocks KCa3.1 in the same manner as TRAM-34 by binding to threonine 250 and valine 275 in the inner pore [41]. Senicapoc is orally bioavailable and has a half-life of 12.8 days in humans [55], which is significantly longer than TRAM-34 that has a half-life of 2 hours in rhesus macaques [56]. Senicapoc’s long half-life in humans is likely the result of reduced oxidative metabolism due to the presence of the two fluorine substituents in para position on two of the phenyl rings [54]. Unfortunately, senicapoc has a much shorter half-live (~1 h) in rodents making it a less optimal tool for animal studies.
Icagen Inc. developed senicapoc as a drug candidate to treat sickle cell disease. Sickle cell disease or sickle cell anemia is a blood disorder caused by a mutation affecting hemoglobin [57]. The mutated hemoglobin (Hb S) has a higher propensity to polymerization, forcing red blood cells into a sickle shape. Due to the unique dependence of Hb S polymerization on intracellular Hb S concentration, preventing erythrocyte dehydration should markedly reduce polymerization. Since K+ efflux through KCa3.1 (= Gárdos channel) is a major pathway for sickle cell dehydration, it made sense to test KCa3.1 inhibitors, which had been shown to prevent erythrocyte dehydration in both patients [38] and transgenic mouse models [58], as treatments for sickle cell disease [57]. The initial Phase II clinical trial seemed to confirm this therapeutic hypothesis by showing that senicapoc was able to reduce hemolysis and increase hemoglobin levels in sickle cell patients [59]. However, in a subsequent Phase III trial [60], senicapoc failed to achieve the primary clinical end point, namely a reduction in the rate of vaso-occlusive pain crisis, despite clearly improving all hematological laboratory parameters, suggesting that erythrocyte dehydration is not the only factor that influences sickling crises. Since senicapoc had been safe and well tolerated in humans, Icagen Inc. explored other therapeutic opportunities for senicapoc after this failure. Based on senicapoc’s efficacy in an asthma model in sheep [61], the compound was tested in two small Phase II trials for asthma [62]. While it demonstrated encouraging results in allergic asthma, it did not improve lung function in exercise induced asthma. Icagen Inc. was subsequently purchased by Pfizer and senicapoc was deposited in the 2012/13 NIH National Center for Advancing Translational Research (NCAT) library as PF-05416266 making it theoretically available for investigator initiated clinical trials. Based on animal studies showing that senicapoc alleviates bleomycin induced pulmonary fibrosis in sheep [63] and reverses tactile allodynia in rats with peripheral nerve injury [64], KCa3.1 since then has been suggested as a potential therapeutic target for idiopathic pulmonary fibrosis (IPF) and neuropathic pain. Since senicapoc is brain penetrant [64] it has also been proposed by two groups for potential repurposing for reducing microglia activation in Alzheimer’s disease and stroke [65, 66]. As recently demonstrated, senicapoc would indeed be suitable for this purpose since it exhibits excellent brain penetration, a property that had not previously been investigated when it was under development for sickle cell anemia. In both mice and rats, senicapoc exhibits roughly 6-fold higher concentrations in the brain than in the blood [52] and is present in the cerebral spinal fluid of rats at free concentration levels that are 2-fold higher than plasma [64]. Oral administration of a senicapoc-medicated chow for 6-months has further been found to mitigate microglia activation in 5xFAD mice and improve hippocampal LTP [52].
1.3. NS6180
NS6180 is a relatively new KCa3.1 blocker identified by scientists at NeuroSearch A/S in a high throughput fluorescence-based Tl+ influx assay [67]. NS6180 represents a novel chemical structure, a benzothiazinone scaffold (Fig. 1), a departure from the triarylmethane-type blockers like TRAM-34 and senicapoc but with the same mechanism of inhibition since the compound also binds to threonine 250 and valine 275 in the pore lumen of KCa3.1 [39] and thus prevents ion permeation in a similar fashion to TRAM-34 [41]. NS6180 blocks KCa3.1 with an IC50 value of 9 nM and has a similarly high, but slightly different selectivity as TRAM-34 [39] and therefore constitutes and attractive alternative pharmacological tool compound for in vitro work. Unfortunately, NS6180 has an extremely low bioavailability and therefore is only suitable for local applications like inflammatory bowel disease [39].
1.4. Other KCa3.1 Inhibitors
While there are other, relatively low potency, KCa3.1 inhibitors which were previously reviewed in detail [68, 69], another interesting, but rarely used class of small molecule KCa3.1 blockers are the 4-phenyl-4H-pyrans [70] and the related cyclohexadienes [71], which were designed by chemists at Bayer. While initial attempts at NeuroSearch A/S to use the L-type calcium channel blocker nifedipine, which had long been known to inhibit KCa3.1 [72], as a template for designing selective KCa3.1 blockers failed, Urbahns et al. hypothesized that isosteric replacement of the NH in nifedipine’s dihydropyridine ring (Fig. 1) with an oxygen might lead to more potent and specific KCa3.1 blockers. This modification together with additional derivatization on the phenyl ring resulted in a 4-Cl, 3-CF3 substituted phenyl-4H-pyran (Fig. 1) that blocked KCa3.1 with an IC50 of 8 nM [70]. A subsequent exchange of O for CH2 and a cyclization of one of the ester groups into a lactone ring resulted in the equally potent cyclohexadienes lactones exemplified by the compound shown in Fig. 1 [71], which exhibits good selectivity over other ion channels, transporters and receptors in binding assays and has been reported to show efficacy in a model of traumatic brain injury [73]. Unfortunately, these interesting compounds seem to have been abandoned when Bayer pulled out of stroke research more than a decade ago and have not been worked with by academic researchers due to the impossibility of obtaining the compounds. Our own group recently resynthesized the phenyl-4H-pyran and found that it indeed blocked KCa3.1 with an IC50 of 8 nM in electrophysiological experiments but was extremely difficult to synthesize [41]. Interestingly, in contrast to its template nifedipine, which is binding in the fenestration region of KCa3.1 (Fig. 2) and might be stabilizing the channel in a non-conducting conformation, the 4-phenyl-pyran is binding in the pore lumen and directly inhibits ion conduction similar to the triarylmethanes TRAM-34 and senicapoc [41]. Having synthesized the phenyl-4H-pyran shown in Fig. 1 (methyl-5-acetyl-4-(4-chloro-3-(trifluoromethyl)phenyl)-2,6-dimethyl-4H-pyran-3-carboxylate) our laboratory also studied its pharmacokinetic properties in mice and found that the compound exhibited good brain penetration (Cbrain/Cplasma ~ 3/1) but that it had a disappointingly short elimination half-life of 15 min following i.v. administration and therefore did not constitute a viable alternative to TRAM-34 and senicapoc for animal work.
1.5. KCa3.1 as a Potential Therapeutic Target for Glioblastoma
As discussed in more detail in the accompanying articles in this thematic issue of Current Neuropharmacology KCa3.1 overexpression in glioma patients is significantly correlated with poor patient survival and invasiveness suggesting KCa3.1 as a target to reduce glioma invasiveness and progression [74]. Encouraging proof-of-concept data for this therapeutic hypothesis was provided by animal experiments demonstrating that both KCa3.1-silencing and treatment with TRAM-34 reduced tumor infiltration as well as microgliosis and astrogliosis in response to tumor-released factors in SCID mice xenografted with human glioma cells [27]. TRAM-34 has further been found to synergize with the methylating agent temozolomide (TMZ), the current standard therapy for glioma, in reducing glioma cell migration, invasion and colony forming activity and increasing the mean survival time in a syngeneic mouse glioma model [75]. Based on these findings, TRAM-34 should constitute a promising compound for treating gliomas. It should of course be kept in mind here that most of these target validating in vitro and in vivo experiments have been performed with established glioma cell lines, which only partially reproduce the cellular heterogeneity present in glioma. It would therefore be desirable if KCa3.1 inhibitors could in future be tested in 3D culture models using primary glioblastoma and cancer stem cells since they have been proposed to more reliably predict drug efficacy and radiation responses [82, 83].
Unfortunately, TRAM-34 has never even entered the long and arduous path of clinical development. Due to its lack of oral availability, which could not be overcome by microencapsulation, its short 2-hour half-life in primates [56], and the expiration of the University of California patent protecting the compound, TRAM-34 has been and will remain a pharmacological tool compound and not a clinically useful drug. However, senicapoc, which as described above has undergone IND enabling toxicity testing and has been previously found to be safe in humans in Phase I, II and III clinical trials [59, 60], would be available for repurposing and had been deposited by Pfizer for exactly this purpose in the NCATs library. Unfortunately, as our own group is currently finding out, there no longer is any drug product suitable for human use. Any academic investigators wanting to perform investigator initiated clinical trials for indications like reducing neuroinflammation in Alzheimer’s disease [65] or stroke [66], treating hereditary xerocytosis, a rare blood disorder caused by a gain-of-function mutation in KCa3.1 [76], or potential adjuvant therapy for glioma, will therefore have to file a new IND with the FDA or the European Medical Agency and have new drug product synthesized and formulated for these trials. Nevertheless, repurposing senicapoc would over all be the fastest way of bringing a KCa3.1 blocker into the clinic despite the fact that it would have been desirable to identify a new compound with a longer remaining patent life, a lower plasma protein binding, an even better brain penetration and a shorter, less variable half-life in patients. However, such a compound would have to undergo both preclinical and clinical development and it would be many years before it would be available to treat glioblastomas.
1.6. KCa3.1 Activators as Potential Anti-cancer Agents?
Similar to many other cancers, adoptive T-cell therapy is being investigated for the treatment of glioblastomas. For example, intracranial infusion of chimeric antigen receptor (CAR)-engineered T cells targeting the glioblastoma-associated antigen interleukin-13 receptor alpha 2 (IL13Rα2) has been shown to successfully induce glioblastoma regression [77]. In this context it should be mentioned that KCa3.1 activators have recently been proposed as potential pharmacological agents for enhancing T cell-mediated tumor clearance [78]. The concept behind this exciting suggestion is that the increased extracellular K+ concentration in the necrotic tumor environment increases intracellular K+ in tumor infiltrating T-cells above an “ionic checkpoint” that suppresses T-cell function within tumors. Genetic manipulation like overexpression of the voltage-gated K+ channel KV1.3 that lower intracellular K+ by enhancing K+ efflux have been shown to improve T cell functions in vitro and enhance tumor clearance and survival in melanoma-bearing mice [79]. Based on these findings pharmacological activation of KCa3.1 has been predicted to have a similar enhancing effect on the antitumor actions of T cells. Since there are currently several KCa3.1 channel activators available like riluzole or the more potent and selective derivatives SKA-31 and SKA-121 [80, 81], this is a very testable hypothesis, for which the ion channel field eagerly awaits the answer. However, assessing KCa3.1 activators in glioblastoma models would be complicated by the fact that KCa3.1 is expressed in glioblastomas, microglia and tumor associated T cell, all of which interact in a highly complex manner.
CONCLUSION
We here examined the usefulness of TRAM-34, senicapoc, NS6180 and the 4-phenyl-4H-pyrans to inhibit KCa3.1 and exert pharmacologic and therapeutic benefit. Out of these compounds, TRAM-34 and senicapoc are of interest for the treatment of glioblastomas because they can cross the blood brain barrier. While TRAM-34 has demonstrated efficacy in reducing glioma invasiveness, glioma associated microgliosis and astrogliosis, and increasing survival time in mouse glioma models [27, 74, 75], and thus has been useful in validating KCa3.1 as a novel pharmacological target for the treatment of glioblastomas, senicapoc, which has previously been in Phase III clinical trials, would be available for repurposing, and could be used to quickly translate the promising findings made with KCa3.1 into clinical reality through investigator initiated clinical trials.
ACKNOWLEDGEMENTS
This work was supported by a National Institute of Neurological Disease and Stroke Award (R56NS098328) to Heike Wulff. Brandon Pressly was supported by a National Institute of General Medical Sciences funded Pharmacology Training Program [T32GM099608].
LIST OF ABBREVIATIONS
- CAR
chimeric antigen receptor
- CRAC
calcium release activated Ca2+ channel
- FDA
Food and Drug Administration
- FGF
fibroblast derived growth factor
- GBM
glioblastoma multiforme
- IND
investigational new drug application
- KCa3.1
intermediate-conductance Ca2+-activated K+ channel
- LTP
long term potentiation
- NCAT
National Center for Advancing Translational Research
- NS6180
(4-[[3-(trifluoromethyl)phenyl]methyl]-2H-1,4-benzothiazin-3(4H)-one)
- PDGF
platelet derived growth factor
- sAHP
slow after hyperpolarization
- SKA-31
naphtho[1,2-d]thiazol-2-ylamine
- SKA-121
5-methylnaphtho[2,1-d]oxazol-2-amine
- Senicapoc
2,2-bis-(4-fluorophenyl)-2-phenylacetamide
- TMZ
temozolomide
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
Consent for Publication
Not applicable.
Conflict of Interest
The University of California patent protecting TRAM-34 and its derivatives as immunosuppressants has been abandoned by the University of California due to its imminent expiration.
REFERENCES
- 1.Bleeker F.E., Molenaar R.J., Leenstra S. Recent advances in the molecular understanding of glioblastoma. J. Neurooncol. 2012;108(1):11–27. doi: 10.1007/s11060-011-0793-0. [http://dx.doi.org/10.1007/s11060-011-0793-0]. [PMID: 22270850]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016;20(5) Suppl.:S2–S8. doi: 10.1188/16.CJON.S1.2-8. [http://dx.doi. org/10.1188/16.CJON.S1.2-8]. [PMID: 27668386]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanger C.M., Ghanshani S., Logsdon N.J., Rauer H., Kalman K., Zhou J., Beckingham K., Chandy K.G., Cahalan M.D., Aiyar J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 1999;274(9):5746–5754. doi: 10.1074/jbc.274.9.5746. [http://dx.doi.org/10.1074/jbc.274.9.5746]. [PMID: 10026195]. [DOI] [PubMed] [Google Scholar]
- 4.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim. Biophys. Acta. 1958;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [http://dx.doi.org/10.1016/0006-3002(58)90124-0]. [PMID: 13618284]. [DOI] [PubMed] [Google Scholar]
- 5.Vandorpe D.H., Shmukler B.E., Jiang L., Lim B., Maylie J., Adelman J.P., de Franceschi L., Cappellini M.D., Brugnara C., Alper S.L. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J. Biol. Chem. 1998;273(34):21542–21553. doi: 10.1074/jbc.273.34.21542. [http://dx.doi.org/10.1074/jbc.273.34.21542]. [PMID: 9705284]. [DOI] [PubMed] [Google Scholar]
- 6.Begenisich T., Nakamoto T., Ovitt C.E., Nehrke K., Brugnara C., Alper S.L., Melvin J.E. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J. Biol. Chem. 2004;279(46):47681–47687. doi: 10.1074/jbc.M409627200. [http://dx.doi.org/10.1074/ jbc.M409627200]. [PMID: 15347667]. [DOI] [PubMed] [Google Scholar]
- 7.Joiner W.J., Wang L.Y., Tang M.D., Kaczmarek L.K. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc. Natl. Acad. Sci. USA. 1997;94(20):11013–11018. doi: 10.1073/pnas.94.20.11013. [http://dx.doi.org/10.1073/pnas.94.20.11013]. [PMID: 9380751]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Si H., Heyken W.T., Wölfle S.E., Tysiac M., Schubert R., Grgic I., Vilianovich L., Giebing G., Maier T., Gross V., Bader M., de Wit C., Hoyer J., Köhler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ. Res. 2006;99(5):537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [http://dx. doi.org/10.1161/01.RES.0000238377.08219.0c]. [PMID: 16873714]. [DOI] [PubMed] [Google Scholar]
- 9.Heitzmann D., Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol. Rev. 2008;88(3):1119–1182. doi: 10.1152/physrev.00020.2007. [http://dx.doi.org/10.1152/physrev.00020. 2007]. [PMID: 18626068]. [DOI] [PubMed] [Google Scholar]
- 10.Ghanshani S., Wulff H., Miller M.J., Rohm H., Neben A., Gutman G.A., Cahalan M.D., Chandy K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [http://dx.doi.org/10.1074/jbc.M003941200]. [PMID: 10961988]. [DOI] [PubMed] [Google Scholar]
- 11.Shumilina E., Lam R.S., Wölbing F., Matzner N., Zemtsova I.M., Sobiesiak M., Mahmud H., Sausbier U., Biedermann T., Ruth P., Sausbier M., Lang F. Blunted IgE-mediated activation of mast cells in mice lacking the Ca2+-activated K+ channel KCa3.1. J. Immunol. 2008;180(12):8040–8047. doi: 10.4049/jimmunol.180.12.8040. [http://dx.doi.org/10.4049/ jimmunol.180.12.8040]. [PMID: 18523267]. [DOI] [PubMed] [Google Scholar]
- 12.Kang H., Kerloc’h A., Rotival M., Xu X., Zhang Q., D’Souza Z., Kim M., Scholz J.C., Ko J.H., Srivastava P.K., Genzen J.R., Cui W., Aitman T.J., Game L., Melvin J.E., Hanidu A., Dimock J., Zheng J., Souza D., Behera A.K., Nabozny G., Cook H.T., Bassett J.H., Williams G.R., Li J., Vignery A., Petretto E., Behmoaras J. Kcnn4 is a regulator of macrophage multinucleation in bone homeostasis and inflammatory disease. Cell Reports. 2014;8(4):1210–1224. doi: 10.1016/j.celrep.2014.07.032. [http://dx.doi.org/10.1016/j.celrep.2014.07.032]. [PMID: 25131209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler R., Wulff H., Eichler I., Kneifel M., Neumann D., Knorr A., Grgic I., Kämpfe D., Si H., Wibawa J., Real R., Borner K., Brakemeier S., Orzechowski H.D., Reusch H.P., Paul M., Chandy K.G., Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108(9):1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [http://dx.doi.org/10.1161/01.CIR.0000086464.04719.DD]. [PMID: 12939222]. [DOI] [PubMed] [Google Scholar]
- 14.Grgic I., Kiss E., Kaistha B.P., Busch C., Kloss M., Sautter J., Müller A., Kaistha A., Schmidt C., Raman G., Wulff H., Strutz F., Gröne H.J., Köhler R., Hoyer J. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc. Natl. Acad. Sci. USA. 2009;106(34):14518–14523. doi: 10.1073/pnas.0903458106. [http://dx.doi.org/10. 1073/pnas.0903458106]. [PMID: 19706538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jäger H., Dreker T., Buck A., Giehl K., Gress T., Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol. Pharmacol. 2004;65(3):630–638. doi: 10.1124/mol.65.3.630. [http://dx.doi.org/10.1124/mol.65.3.630]. [PMID: 14978241]. [DOI] [PubMed] [Google Scholar]
- 16.Parihar A.S., Coghlan M.J., Gopalakrishnan M., Shieh C.C. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur. J. Pharmacol. 2003;471(3):157–164. doi: 10.1016/s0014-2999(03)01825-9. [http://dx.doi.org/10.1016/ S0014-2999(03)01825-9]. [PMID: 12826234]. [DOI] [PubMed] [Google Scholar]
- 17.Grössinger E.M., Weiss L., Zierler S., Rebhandl S., Krenn P.W., Hinterseer E., Schmölzer J., Asslaber D., Hainzl S., Neureiter D., Egle A., Piñón-Hofbauer J., Hartmann T.N., Greil R., Kerschbaum H.H. Targeting proliferation of chronic lymphocytic leukemia (CLL) cells through KCa3.1 blockade. Leukemia. 2014;28(4):954–958. doi: 10.1038/leu.2014.37. [http://dx.doi.org/10.1038/leu.2014.37]. [PMID: 24441290]. [DOI] [PubMed] [Google Scholar]
- 18.Cahalan M.D., Chandy K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009;231(1):59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [http:// dx.doi.org/10.1111/j.1600-065X.2009.00816.x]. [PMID: 19754890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feske S., Wulff H., Skolnik E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [http:// dx.doi.org/10.1146/annurev-immunol-032414-112212]. [PMID: 25861976]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushal V., Koeberle P.D., Wang Y., Schlichter L.C. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 2007;27(1):234–244. doi: 10.1523/JNEUROSCI.3593-06.2007. [http://dx.doi.org/10.1523/JNEUROSCI. 3593-06.2007]. [PMID: 17202491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maezawa I., Zimin P.I., Wulff H., Jin L.W. Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 2011;286(5):3693–3706. doi: 10.1074/jbc.M110.135244. [http://dx.doi.org/10.1074/jbc.M110.135244]. [PMID: 20971854]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen H.M., Grössinger E.M., Horiuchi M., Davis K.W., Jin L.W., Maezawa I., Wulff H. Differential Kv1.3, KCa3.1, and Kir2.1 expression in “classically” and “alternatively” activated microglia. Glia. 2017;65(1):106–121. doi: 10.1002/glia.23078. [http://dx.doi.org/10.1002/glia. 23078]. [PMID: 27696527]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y.J., Nguyen H.M., Maezawa I., Grössinger E.M., Garing A.L., Köhler R., Jin L.W., Wulff H. The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 2016;36(12):2146–2161. doi: 10.1177/0271678X15611434. [http://dx.doi.org/10.1177/ 0271678X15611434]. [PMID: 26661208]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King B., Rizwan A.P., Asmara H., Heath N.C., Engbers J.D., Dykstra S., Bartoletti T.M., Hameed S., Zamponi G.W., Turner R.W. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Reports. 2015;11(2):175–182. doi: 10.1016/j.celrep.2015.03.026. [http://dx.doi.org/10.1016/j.celrep.2015.03.026]. [PMID: 25865881]. [DOI] [PubMed] [Google Scholar]
- 25.Wang K., Mateos-Aparicio P., Hönigsperger C., Raghuram V., Wu W.W., Ridder M.C., Sah P., Maylie J., Storm J.F., Adelman J.P. IK1 channels do not contribute to the slow afterhyperpolarization in pyramidal neurons. eLife. 2016;5:e11206. doi: 10.7554/eLife.11206. [PMID: 26765773]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catacuzzeno L., Fioretti B., Franciolini F. Expression and role of the intermediate-conductance calcium-activated potassium channel KCa3.1 in glioblastoma. J. Signal Transduct. 2012;2012:421564. doi: 10.1155/2012/421564. [http://dx.doi.org/10.1155/2012/421564]. [PMID: 22675627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Alessandro G., Catalano M., Sciaccaluga M., Chece G., Cipriani R., Rosito M., Grimaldi A., Lauro C., Cantore G., Santoro A., Fioretti B., Franciolini F., Wulff H., Limatola C. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013;4:e773. doi: 10.1038/cddis.2013.279. [http://dx. doi.org/10.1038/cddis.2013.279]. [PMID: 23949222]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimaldi A., D’Alessandro G., Golia M.T., Grössinger E.M., Di Angelantonio S., Ragozzino D., Santoro A., Esposito V., Wulff H., Catalano M., Limatola C. KCa3.1 inhibition switches the phenotype of glioma-infiltrating microglia/macrophages. Cell Death Dis. 2016;7:e2174. doi: 10.1038/cddis.2016.73. [http://dx.doi.org/10.1038/cddis.2016.73]. [PMID: 27054329]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauer H., Lanigan M.D., Pennington M.W., Aiyar J., Ghanshani S., Cahalan M.D., Norton R.S., Chandy K.G. Structure-guided transformation of charybdotoxin yields an analog that selectively targets Ca(2+)-activated over voltage-gated K(+) channels. J. Biol. Chem. 2000;275(2):1201–1208. doi: 10.1074/jbc.275.2.1201. [http://dx.doi.org/10.1074/ jbc.275.2.1201]. [PMID: 10625664]. [DOI] [PubMed] [Google Scholar]
- 30.Kharrat R., Mabrouk K., Crest M., Darbon H., Oughideni R., Martin-Eauclaire M.F., Jacquet G., el Ayeb M., Van Rietschoten J., Rochat H., Sabatier J.M. Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur. J. Biochem. 1996;242(3):491–498. doi: 10.1111/j.1432-1033.1996.0491r.x. [http://dx.doi.org/10.1111/j.1432-1033.1996.0491r.x]. [PMID: 9022673]. [DOI] [PubMed] [Google Scholar]
- 31.Castle N.A., London D.O., Creech C., Fajloun Z., Stocker J.W., Sabatier J-M. Maurotoxin: a potent inhibitor of intermediate conductance Ca2+-activated potassium channels. Mol. Pharmacol. 2003;63(2):409–418. doi: 10.1124/mol.63.2.409. [http://dx.doi.org/10.1124/mol.63.2.409]. [PMID: 12527813]. [DOI] [PubMed] [Google Scholar]
- 32.Wulff H., Miller M.J., Hansel W., Grissmer S., Cahalan M.D., Chandy K.G. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc. Natl. Acad. Sci. USA. 2000;97(14):8151–8156. doi: 10.1073/pnas.97.14.8151. [http://dx.doi.org/10.1073/pnas.97.14.8151]. [PMID: 10884437]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brugnara C., de Franceschi L., Alper S.L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Invest. 1993;92(1):520–526. doi: 10.1172/JCI116597. [http://dx.doi.org/10.1172/JCI116597]. [PMID: 8326017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki S., Kurata N., Nishimura Y., Yasuhara H., Satoh T. Effects of imidazole antimycotics on the liver microsomal cytochrome P450 isoforms in rats: comparison of in vitro and ex vivo studies. Eur. J. Drug Metab. Pharmacokinet. 2000;25(2):121–126. doi: 10.1007/BF03190078. [http://dx.doi.org/10.1007/BF03190078]. [PMID: 11112093]. [DOI] [PubMed] [Google Scholar]
- 35.Luo G., Cunningham M., Kim S., Burn T., Lin J., Sinz M., Hamilton G., Rizzo C., Jolley S., Gilbert D., Downey A., Mudra D., Graham R., Carroll K., Xie J., Madan A., Parkinson A., Christ D., Selling B., LeCluyse E., Gan L.S. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab. Dispos. 2002;30(7):795–804. doi: 10.1124/dmd.30.7.795. [http://dx.doi.org/10.1124/dmd. 30.7.795]. [PMID: 12065438]. [DOI] [PubMed] [Google Scholar]
- 36.Benzaquen L.R., Brugnara C., Byers H.R., Gatton-Celli S., Halperin J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995;1(6):534–540. doi: 10.1038/nm0695-534. [http://dx.doi.org/10.1038/ nm0695-534]. [PMID: 7585119]. [DOI] [PubMed] [Google Scholar]
- 37.de Franceschi L., Rouyer-Fessard P., Alper S.L., Jouault H., Brugnara C., Beuzard Y. Combination therapy of erythropoietin, hydroxyurea, and clotrimazole in a beta thalassemic mouse: a model for human therapy. Blood. 1996;87(3):1188–1195. [PMID: 8562946]. [PubMed] [Google Scholar]
- 38.Brugnara C., Gee B., Armsby C.C., Kurth S., Sakamoto M., Rifai N., Alper S.L., Platt O.S. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J. Clin. Invest. 1996;97(5):1227–1234. doi: 10.1172/JCI118537. [http://dx.doi.org/10.1172/JCI118537]. [PMID: 8636434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strøbæk D., Brown D.T., Jenkins D.P., Chen Y.J., Coleman N., Ando Y., Chiu P., Jørgensen S., Demnitz J., Wulff H., Christophersen P. NS6180, a new K(Ca) 3.1 channel inhibitor prevents T-cell activation and inflammation in a rat model of inflammatory bowel disease. Br. J. Pharmacol. 2013;168(2):432–444. doi: 10.1111/j.1476-5381.2012.02143.x. [http:// dx.doi.org/10.1111/j.1476-5381.2012.02143.x]. [PMID: 22891655]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wulff H., Gutman G.A., Cahalan M.D., Chandy K.G. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J. Biol. Chem. 2001;276(34):32040–32045. doi: 10.1074/jbc.M105231200. [http://dx.doi.org/10.1074/ jbc.M105231200]. [PMID: 11425865]. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen H.M., Singh V., Pressly B., Jenkins D.P., Wulff H., Yarov-Yarovoy V. Structural Insights into the atomistic mechanisms of action of small molecule inhibitors targeting the KCa3.1 channel pore. Mol. Pharmacol. 2017;91(4):392–402. doi: 10.1124/mol.116.108068. [http://dx. doi.org/10.1124/mol.116.108068]. [PMID: 28126850]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y.J., Raman G., Bodendiek S., O’Donnell M.E., Wulff H. The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 2011;31(12):2363–2374. doi: 10.1038/jcbfm.2011.101. [http://dx.doi.org/10. 1038/jcbfm.2011.101]. [PMID: 21750563]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyama K., Wulff H., Chandy K.G., Azam P., Raman G., Saito T., Fujiwara Y., Mattson D.L., Das S., Melvin J.E., Pratt P.F., Hatoum O.A., Gutterman D.D., Harder D.R., Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J. Clin. Invest. 2008;118(9):3025–3037. doi: 10.1172/JCI30836. [http://dx.doi.org/10.1172/ JCI30836]. [PMID: 18688283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tharp D.L., Wamhoff B.R., Wulff H., Raman G., Cheong A., Bowles D.K. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler. Thromb. Vasc. Biol. 2008;28(6):1084–1089. doi: 10.1161/ATVBAHA.107.155796. [http://dx.doi.org/10.1161/ ATVBAHA.107.155796]. [PMID: 18309114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girodet P.O., Ozier A., Carvalho G., Ilina O., Ousova O., Gadeau A.P., Begueret H., Wulff H., Marthan R., Bradding P., Berger P. Ca(2+)-activated K(+) channel-3.1 blocker TRAM-34 attenuates airway remodeling and eosinophilia in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 2013;48(2):212–219. doi: 10.1165/rcmb.2012-0103OC. [http:// dx.doi.org/10.1165/rcmb.2012-0103OC]. [PMID: 23204391]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y.J., Lam J., Gregory C.R., Schrepfer S., Wulff H. The Ca2+-activated K+ channel KCa3.1 as a potential new target for the prevention of allograft vasculopathy. PLoS One. 2013;8(11):e81006. doi: 10.1371/journal.pone.0081006. [http://dx.doi.org/10.1371/journal.pone.0081006]. [PMID: 24312257]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hua X., Deuse T., Chen Y.J., Wulff H., Stubbendorff M., Köhler R., Miura H., Länger F., Reichenspurner H., Robbins R.C., Schrepfer S. The potassium channel KCa3.1 as new therapeutic target for the prevention of obliterative airway disease. Transplantation. 2013;95(2):285–292. doi: 10.1097/TP.0b013e318275a2f4. [http://dx.doi.org/10.1097/ TP.0b013e318275a2f4]. [PMID: 23325003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di L., Srivastava S., Zhdanova O., Ding Y., Li Z., Wulff H., Lafaille M., Skolnik E.Y. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc. Natl. Acad. Sci. USA. 2010;107(4):1541–1546. doi: 10.1073/pnas.0910133107. [http://dx.doi.org/10.1073/pnas.0910133107]. [PMID: 20080610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C., Shen S., Ma Q., Chen J., Gill A., Pollock C.A., Chen X.M. Blockade of KCa3.1 ameliorates renal fibrosis through the TGF-β1/Smad pathway in diabetic mice. Diabetes. 2013;62(8):2923–2934. doi: 10.2337/db13-0135. [http://dx.doi.org/10.2337/db13-0135]. [PMID: 23656889]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ju C.H., Wang X.P., Gao C.Y., Zhang S.X., Ma X.H., Liu C. Blockade of KCa3.1 attenuates left ventricular remodeling after experimental myocardial infarction. Cell. Physiol. Biochem. 2015;36(4):1305–1315. doi: 10.1159/000430298. [http://dx.doi.org/10.1159/000430298]. [PMID: 26160442]. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L.M., Wang L.P., Wang H.F., Ma X.Z., Zhou D.X., Deng X.L. The role of KCa3.1 channels in cardiac fibrosis induced by pressure overload in rats. Pflugers Arch. 2015;467(11):2275–2285. doi: 10.1007/s00424-015-1694-4. [http://dx.doi.org/10.1007/s00424-015-1694-4]. [PMID: 25715999]. [DOI] [PubMed] [Google Scholar]
- 52.Maezawa I., Nguyen H.M., DiLucente J., Singh V., Chavez M., Wulff H., Jin L.W. 2016. [Google Scholar]
- 53.Stocker J.W., De Franceschi L., McNaughton-Smith G.A., Corrocher R., Beuzard Y., Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101(6):2412–2418. doi: 10.1182/blood-2002-05-1433. [http:// dx.doi.org/10.1182/blood-2002-05-1433]. [PMID: 12433690]. [DOI] [PubMed] [Google Scholar]
- 54.McNaughton-Smith G.A., Burns J.F., Stocker J.W., Rigdon G.C., Creech C., Arrington S., Shelton T., de Franceschi L. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J. Med. Chem. 2008;51(4):976–982. doi: 10.1021/jm070663s. [http://dx.doi. org/10.1021/jm070663s]. [PMID: 18232633]. [DOI] [PubMed] [Google Scholar]
- 55.Ataga K.I., Orringer E.P., Styles L., Vichinsky E.P., Swerdlow P., Davis G.A., Desimone P.A., Stocker J.W. Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy. 2006;26(11):1557–1564. doi: 10.1592/phco.26.11.1557. [http://dx.doi.org/10.1592/ phco.26.11.1557]. [PMID: 17064199]. [DOI] [PubMed] [Google Scholar]
- 56.Abbassi M., Shresta S., Raman G., Wulff H., Pereira L., Ansari A., Al-Ghananeem A. Formulation-based approach to support early drug discovery and development efforts: a case study for entereric microencapsulation of a novel immunosuppressant TRAM-34. Drug Dev. Ind. Pharm. 2010;36:563–569. doi: 10.3109/03639040903329554. [http://dx.doi.org/ 10.3109/03639040903329554]. [PMID: 19929567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brugnara C. Sickle cell disease: from membrane pathophysiology to novel therapies for prevention of erythrocyte dehydration. J. Pediatr. Hematol. Oncol. 2003;25(12):927–933. doi: 10.1097/00043426-200312000-00004. [http://dx.doi.org/ 10.1097/00043426-200312000-00004]. [PMID: 14663274]. [DOI] [PubMed] [Google Scholar]
- 58.De Franceschi L., Saadane N., Trudel M., Alper S.L., Brugnara C., Beuzard Y. Treatment with oral clotrimazole blocks Ca(2+)-activated K+ transport and reverses erythrocyte dehydration in transgenic SAD mice. A model for therapy of sickle cell disease. J. Clin. Invest. 1994;93(4):1670–1676. doi: 10.1172/JCI117149. [http://dx.doi.org/10.1172/ JCI117149]. [PMID: 7512989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ataga K.I., Smith W.R., De Castro L.M., Swerdlow P., Saunthararajah Y., Castro O., Vichinsky E., Kutlar A., Orringer E.P., Rigdon G.C., Stocker J.W. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111(8):3991–3997. doi: 10.1182/blood-2007-08-110098. [http://dx.doi.org/10. 1182/blood-2007-08-110098]. [PMID: 18192510]. [DOI] [PubMed] [Google Scholar]
- 60.Ataga K.I., Reid M., Ballas S.K., Yasin Z., Bigelow C., James L.S., Smith W.R., Galacteros F., Kutlar A., Hull J.H., Stocker J.W. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br. J. Haematol. 2011;153(1):92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [http://dx.doi.org/10.1111/j.1365-2141.2010.08520.x]. [PMID: 21323872]. [DOI] [PubMed] [Google Scholar]
- 61.Van Der Velden J., Sum G., Barker D., Koumoundouros E., Barcham G., Wulff H., Castle N., Bradding P., Snibson K.K. (Ca)3.1 channel-blockade attenuates airway pathophysiology in a sheep model of chronic asthma. PLoS One. 2013;8(6):e66886. doi: 10.1371/journal.pone.0066886. [http://dx.doi.org/10.1371/journal.pone.0066886]. [PMID: 23826167]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wulff H., Castle N.A. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev. Clin. Pharmacol. 2010;3(3):385–396. doi: 10.1586/ecp.10.11. [http://dx.doi.org/10.1586/ecp.10.11]. [PMID: 22111618]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Organ L., Bacci B., Koumoundouros E., Kimpton W.G., Samuel C.S., Nowell C.J., Bradding P., Roach K.M., Westall G., Jaffar J., Snibson K.J. Inhibition of the KCa3.1 channel alleviates established pulmonary fibrosis in a large animal model. Am. J. Respir. Cell Mol. Biol. 2017;56(4):539–550. doi: 10.1165/rcmb.2016-0092OC. [http://dx.doi.org/10. 1165/rcmb.2016-0092OC]. [PMID: 28060543]. [DOI] [PubMed] [Google Scholar]
- 64.Staal R.G.W., Khayrullina T., Zhang H., Davis S., Fallon S.M., Cajina M., Nattini M.E., Hu A., Zhou H., Poda S.B., Zorn S., Chandrasena G., Dale E., Cambpell B., Biilmann Rønn L.C., Munro G., Mӧller T. Inhibition of the potassium channel KCa3.1 by senicapoc reverses tactile allodynia in rats with peripheral nerve injury. Eur. J. Pharmacol. 2017;795:1–7. doi: 10.1016/j.ejphar.2016.11.031. [http://dx.doi.org/ 10.1016/j.ejphar.2016.11.031]. [PMID: 27876619]. [DOI] [PubMed] [Google Scholar]
- 65.Maezawa I., Jenkins D.P., Jin B.E., Wulff H. Microglial KCa3.1 channels as a potential therapeutic target for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012;2012:868972. doi: 10.1155/2012/868972. [http://dx.doi.org/ 10.1155/2012/868972]. [PMID: 22675649]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dale E., Staal R.G., Eder C., Möller T. KCa 3.1-a microglial target ready for drug repurposing? Glia. 2016;64(10):1733–1741. doi: 10.1002/glia.22992. [http://dx.doi.org/10.1002/glia.22992]. [PMID: 27121595]. [DOI] [PubMed] [Google Scholar]
- 67.Jørgensen S., Dyhring T., Brown D.T., Strøbæk D., Christophersen P., Demnitz J. A high-throughput screening campaign for detection of ca(2+)-activated k(+) channel activators and inhibitors using a fluorometric imaging plate reader-based tl(+)-influx assay. Assay Drug Dev. Technol. 2013;11(3):163–172. doi: 10.1089/adt.2012.479. [http://dx.doi. org/10.1089/adt.2012.479]. [PMID: 23198866]. [DOI] [PubMed] [Google Scholar]
- 68.Wulff H., Kolski-Andreaco A., Sankaranarayanan A., Sabatier J.M., Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr. Med. Chem. 2007;14(13):1437–1457. doi: 10.2174/092986707780831186. [http:// dx.doi.org/10.2174/092986707780831186]. [PMID: 17584055]. [DOI] [PubMed] [Google Scholar]
- 69.Wulff H., Zhorov B.S.K.K. + channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem. Rev. 2008;108(5):1744–1773. doi: 10.1021/cr078234p. [http://dx.doi.org/10.1021/cr078234p]. [PMID: 18476673]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urbahns K., Horváth E., Stasch J.P., Mauler F. 4-Phenyl-4H-pyrans as IK(Ca) channel blockers. Bioorg. Med. Chem. Lett. 2003;13(16):2637–2639. doi: 10.1016/s0960-894x(03)00560-2. [http://dx.doi.org/10.1016/S0960-894X (03)00560-2]. [PMID: 12873483]. [DOI] [PubMed] [Google Scholar]
- 71.Urbahns K., Goldmann S., Krüger J., Horváth E., Schuhmacher J., Grosser R., Hinz V., Mauler F. IKCa-channel blockers. Part 2: discovery of cyclohexadienes. Bioorg. Med. Chem. Lett. 2005;15(2):401–404. doi: 10.1016/j.bmcl.2004.10.063. [http://dx.doi.org/10.1016/j.bmcl.2004.10.063]. [PMID: 15603962]. [DOI] [PubMed] [Google Scholar]
- 72.Kaji D.M. Nifedipine inhibits calcium-activated K transport in human erythrocytes. Am. J. Physiol. 1990;259(2 Pt 1):C332–C339. doi: 10.1152/ajpcell.1990.259.2.C332. [http://dx.doi.org/10.1152/ajpcell.1990.259.2.C332]. [PMID: 2382706]. [DOI] [PubMed] [Google Scholar]
- 73.Mauler F., Hinz V., Horváth E., Schuhmacher J., Hofmann H.A., Wirtz S., Hahn M.G., Urbahns K. Selective intermediate-/small-conductance calcium-activated potassium channel (KCNN4) blockers are potent and effective therapeutics in experimental brain oedema and traumatic brain injury caused by acute subdural haematoma. Eur. J. Neurosci. 2004;20(7):1761–1768. doi: 10.1111/j.1460-9568.2004.03615.x. [http://dx.doi. org/10.1111/j.1460-9568.2004.03615.x]. [PMID: 15379997]. [DOI] [PubMed] [Google Scholar]
- 74.Turner K.L., Honasoge A., Robert S.M., McFerrin M.M., Sontheimer H. A proinvasive role for the Ca(2+) -activated K(+) channel KCa3.1 in malignant glioma. Glia. 2014;62(6):971–981. doi: 10.1002/glia.22655. [http://dx.doi.org/10.1002/glia.22655]. [PMID: 24585442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D’Alessandro G., Grimaldi A., Chece G., Porzia A., Esposito V., Santoro A., Salvati M., Mainiero F., Ragozzino D., Di Angelantonio S., Wulff H., Catalano M., Limatola C. KCa3.1 channel inhibition sensitizes malignant gliomas to temozolomide treatment. Oncotarget. 2016;7(21):30781–30796. doi: 10.18632/oncotarget.8761. [http://dx.doi.org/ 10.18632/oncotarget.8761]. [PMID: 27096953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rivera A., Vandorpe D.H., Shmukler B.E., Gallagher D.R., Fikry C.C., Kuypers F.A., Brugnara C., Snyder L.M., Alper S.L. Erythrocytes from hereditary xerocytosis patients heterozygous for KCNN4 V282M exhibit increased spontaneous Gardos channel-like activity inhibited by senicapoc. Am. J. Hematol. 2017;92(6):E108–E110. doi: 10.1002/ajh.24716. Epub ahead of print [http://dx.doi.org/10.1002/ ajh.24716]. [PMID: 28295477]. [DOI] [PubMed] [Google Scholar]
- 77.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., Kurien A., Priceman S.J., Wang X., Harshbarger T.L., D’Apuzzo M., Ressler J.A., Jensen M.C., Barish M.E., Chen M., Portnow J., Forman S.J., Badie B. Regression of glioblastoma after ghimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [http://dx.doi.org/10.1056/ NEJMoa1610497]. [PMID: 28029927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chandy K.G., Norton R.S. Immunology: Channelling potassium to fight cancer. Nature. 2016;537(7621):497–499. doi: 10.1038/nature19467. [http://dx.doi. org/10.1038/nature19467]. [PMID: 27626384]. [DOI] [PubMed] [Google Scholar]
- 79.Eil R., Vodnala S.K., Clever D., Klebanoff C.A., Sukumar M., Pan J.H., Palmer D.C., Gros A., Yamamoto T.N., Patel S.J., Guittard G.C., Yu Z., Carbonaro V., Okkenhaug K., Schrump D.S., Linehan W.M., Roychoudhuri R., Restifo N.P. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537(7621):539–543. doi: 10.1038/nature19364. [http://dx.doi.org/10.1038/nature19364]. [PMID: 27626381]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sankaranarayanan A., Raman G., Busch C., Schultz T., Zimin P.I., Hoyer J., Köhler R., Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol. Pharmacol. 2009;75(2):281–295. doi: 10.1124/mol.108.051425. [http://dx.doi.org/10.1124/mol.108.051425]. [PMID: 18955585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coleman N., Brown B.M., Oliván-Viguera A., Singh V., Olmstead M.M., Valero M.S., Köhler R., Wulff H. New positive Ca2+-activated K+ channel gating modulators with selectivity for KCa3.1. Mol. Pharmacol. 2014;86(3):342–357. doi: 10.1124/mol.114.093286. [http://dx.doi. org/10.1124/mol.114.093286]. [PMID: 24958817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma N.K., Lim J.K., Leong M.F., Sandanaraj E., Ang B.T., Tang C., Wan A.C. Collaboration of 3D context and extracellular matrix in the development of glioma stemness in a 3D model. Biomaterials. 2016;78:62–73. doi: 10.1016/j.biomaterials.2015.11.031. [http://dx.doi.org/10.1016/ j.biomaterials.2015.11.031]. [PMID: 26684838]. [DOI] [PubMed] [Google Scholar]
- 83.Gomez-Roman N., Stevenson K., Gilmour L., Hamilton G., Chalmers A.J. A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses. Neuro-oncol. 2017;19(2):229–241. doi: 10.1093/neuonc/now164. [PMID: 27576873]. [DOI] [PMC free article] [PubMed] [Google Scholar]