Fig. 7. The level of cytoplasmic p53 protein correlates the level of mitochondrial Lon in oral cancer.
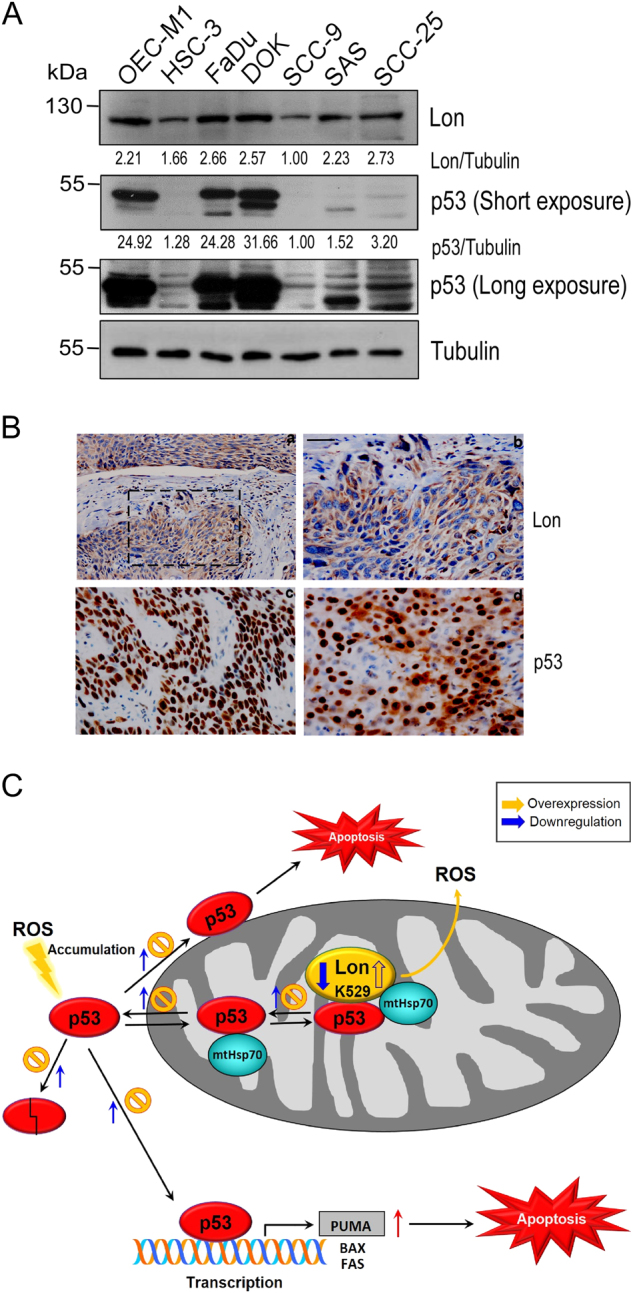
a The protein level of Lon and p53 in oral cancer cell lines. The extracts of oral cancer cell lines were immunoblotted with the indicated antibodies and antibody to Tubulin as a loading control. b Immunohistochemical analysis of Lon and p53 expression in OSCC patients. Representative immunohistochemical analysis of Lon and p53 was performed by using paraffin-embedded sections of OSCC. The representative results shown here are positive staining of Lon (a, 200×; b, 400×), nuclear staining of p53 (c), and nuclear/cytoplasmic staining of p53 (d) in oral cancer tissues. The microscopic magnification of p53 staining was 400×. Scale bar, 50 μm. c Model of p53 accumulation in mitochondria and apoptosis inhibition by chaperone Lon in cancer cells. Upon Lon overexpression and/or oxidative stress, mitochondrial Lon binds with p53 and induces the accumulation of p53 in the matrix through its chaperone activity (residue K529) and inhibits p53-mediated apoptosis including transcription-independent and -dependent mechanisms. Mitochondrial Lon sequesters p53 to inhibit the opening of MPTP on the outer membrane and the MPTP–cyclophilin D complex on the inner membrane. Meanwhile, mitochondrial Lon retains p53 in the matrix to reduce the transcription-dependent function of nuclear distribution of p53. The stability/level of cytosolic p53 is increased by the prevention of proteasome-dependent degradation through sequestering p53 by Lon-mtHsp70 in mitochondrial matrix
