Abstract
Background: The chemopreventive activities of cruciferous vegetables were recognized in the early 1990s, followed by a growth of evidence in various cancer models, including breast cancer. To our knowledge, no studies have examined whether consumption of cruciferous vegetables has changed accordingly, and what impact, if any, on breast cancer risk may have resulted.
Objective: The time trend in cruciferous vegetable intake was investigated between 1982 and 1998, and its associations with breast cancer risk were examined.
Methods: In a hospital-based case-control study in 1491 patients with breast cancer and 1482 controls, loess curves were constructed to describe the relation between median consumption of cruciferous vegetables and year of admission. ORs and 95% CIs were calculated with unconditional logistic regression, adjusting for age, year of admission, family income, body mass index, cigarette smoking, age at menarche, parity, age at first birth, family history of breast cancer, hormone replacement therapy, and total meat intake.
Results: Consumption patterns differed between cases and controls. A slow but steady increase in cruciferous vegetable intake was observed in the cases, although among controls, cruciferous vegetable consumption increased from 1982 to 1987, reached a plateau during 1988–1992, and then declined from 1993 to 1998. Accordingly, although an overall inverse association with breast cancer risk was observed for cruciferous vegetable intake (highest compared with lowest quartile—OR: 0.68; 95% CI: 0.55, 0.86; P-trend = 0.0006), the inverse association tended to be more pronounced within more recent-year strata, with an OR of 0.52 (95% CI: 0.33, 0.83) for 1993–1998 compared with an OR of 0.89 (95% CI: 0.64, 1.23) for 1982–1987.
Conclusions: The consumption of cruciferous vegetables increased during the past 2 decades, showing different trends in cases and controls. The subtle but sustained increase in cruciferous vegetable intake reported by the cases could influence association studies with breast cancer risk.
Keywords: breast cancer, cruciferous vegetable, self-reported consumption, risk association, case-control study
Introduction
Breast cancer is the most common cancer and is the second leading cause of cancer-related mortality among American women (1). Various risk factors and prognostic factors for breast cancer have been studied. The chemopreventive activities of cruciferous vegetables were recognized in the early 1990s, followed by a rapid growth of evidence of their potential preventive role against a number of cancers, including breast cancer (2–5). Cruciferous vegetables are rich unique sources of glucosinolates, whose hydrolytic products, primarily isothiocyanates (ITCs) and indoles, may have cancer chemopreventive properties. Evidence from in vitro and in vivo studies suggests that ITCs and indoles may prevent or inhibit breast cancer development through the following mechanisms: modulating activity of phase I and phase II enzymes (6–8), inhibiting cell proliferation (9–14), regulating the expression of estrogen receptor (15), altering the metabolism of estrogen (16–19), or suppressing cyclooxygenase 2 (COX-2) (20, 21).
Despite strong evidence from cell and animal experiments on the preventive effects of cruciferous vegetables on breast carcinogenesis, epidemiologic studies often generate inconsistent results. In a case-control study, we previously found a marginally significant inverse association with cruciferous vegetable intake among premenopausal women only (OR: 0.60; 95% CI: 0.40, 1.01; P = 0.058) (22), whereas Gaudet et al. (23) reported that reduced risk was only observed among postmenopausal women (OR: 0.80; 95% CI: 0.60, 1.05). Two case-control studies from Chinese populations also presented mixed results. A significant inverse association was observed by Zhang et al. (24) (OR: 0.49; 95% CI: 0.32, 0.74; P < 0.001), whereas Shannon et al. (25) observed no associations, even in the highest intake category (OR: 1.08; 95% CI: 0.62, 1.89). In a meta-analysis including 11 case-control and 2 cohort studies, Liu and Lv (26) found the significant inverse association only among postmenopausal women.
Several factors may contribute to the observed inconsistencies. First, hydrolysis products of glucosinolates, especially ITCs, may be largely reduced by cooking procedures (27–29), which should be taken into account in considering the role of cruciferous vegetables in cancer prevention. Second, cruciferous vegetables were not assessed comprehensively in most of the previous studies. Third, with the increased attention on cruciferous vegetables for cancer prevention, as well as the public health campaigns on vegetable and fruit consumption since the 1990s (30), cruciferous vegetable consumption may have changed considerably, resulting in differential impact on risk-association studies across time. In the current study, we investigated temporal trends in self-reported cruciferous vegetable consumption among breast cancer cases and controls, compared with the trends in total vegetable consumption and examined the effects of cruciferous vegetable intake on breast cancer risk within each time period. These analyses allowed for the consideration of raw compared with cooked consumption separately and included more comprehensive information on cruciferous vegetables commonly consumed in the United States (i.e., broccoli, cabbage, cauliflower, Brussels sprouts, kale, and turnip, collard, and mustard greens).
Methods
Study population
In this case-control study, all cases and controls were drawn from patients at Roswell Park Cancer Institute (RPCI; Buffalo, New York) who participated in the Patient Epidemiology Data System (PEDS) from 1982 to 1998. PEDS contains data gathered from a self-administered survey offered to all new patients receiving medical service at RPCI. Controls were randomly selected from a pool of >8000 potentially eligible patients who came to RPCI with a suspicion of neoplastic disease but were diagnosed with conditions other than cancer and treated at RPCI, including infectious and parasitic diseases (20%), diseases of the circulatory system (12%), ill-defined signs and symptoms (17%), diseases of the genitourinary system (13%), benign neoplasms (8%), and other various conditions (28%) during the same time period as were cases. The overall response rate was ∼50% for both cases and controls. Participants provided written informed consent. Procedures for the protection of human subjects in this study were approved by the Institutional Review Board at RPCI. Breast cancer cases in the current study were defined as women diagnosed with incident, primary, histologically confirmed breast cancer. Controls were eligible if they were cancer-free at the time of service, without benign tumors, and with no previous history of cancer. Controls were frequency matched to cases on age and year of admission to the PEDS study (usually same as year of disease diagnosis) by using 5-y intervals for both of these criteria. In total, 1491 breast cancer cases and 1482 controls who were predominantly white (99%) and ranged in age from 21 to 97 y were included.
Questionnaire data
Data relevant to breast cancer risk factors were obtained for cases and controls from the PEDS questionnaires, which included information on demographic characteristics, tobacco use and alcohol consumption, reproductive history, medical history, and family history of cancer, as well as a 44-item FFQ assessing usual diet in the few years before diagnosis. The 44-item FFQ was designed to provide an assessment of intakes of fruit and vegetables, including cruciferous vegetables, and foods that are sources of fat, fiber, and vitamins A, C, and E (31). This brief FFQ was validated by Byers et al. (31) with the use of data from the Western New York Diet Study, showing that a large fraction of the variability in nutrient intake in the population of western New York regions could be explained by a small number of foods ascertained in an abbreviated dietary history questionnaire for epidemiologic studies. For cruciferous vegetables, the consumption of broccoli, cabbage, and cauliflower was documented separately as raw or cooked, whereas intakes of other vegetables, including Brussels sprouts, kale, turnip greens, collard greens, and mustard greens, were combined intakes of both raw and cooked. The consumption of each vegetable was queried according to the categories of never, <1 time/mo, 1–3 times/mo, 1–4 times/wk, or 5–7 times/wk, and a serving size of 0.5 cup was applied. Only the participants with all categories of cruciferous vegetable intake missing were excluded from the analysis.
The monthly frequency of consumption for each food category was calculated, and the unit denoted as servings per month (1 serving = 0.5 cup). Intakes of kale and turnip, collard, and mustard greens were summed as “greens & kale” intake, because their individual consumption is relatively uncommon. Total cruciferous vegetable intake was calculated as the sum intake of all individual cruciferous vegetables in our study. Total vegetable intake was calculated as the sum of carrots, tomatoes, spinach, lettuce, beans, white potatoes, squash (soft or hard), green peas, eggplant, tomato juice, and total cruciferous vegetables. Total energy intake could not be calculated from the brief 44-item FFQ; therefore, total meat intake was used to adjust for the potential confounding effect of overall diet composition. Total meat intake was the sum intake of pork chops, hotdogs, canned ham, ham, salami, liver, pork sausage, beef, bacon, chicken, and hamburger.
Statistical analysis
On the basis of the distribution in the controls, the intake of total cruciferous vegetables was categorized into quartiles and broccoli, cabbage, and cauliflower intakes were categorized into tertiles. For individual cruciferous vegetables in both raw and cooked categories, as well as Brussels sprouts and “greens & kale,” consumption was dichotomized (<1 and ≥1 servings/mo). Menopausal status was self-reported. Women without information on their menopausal status (11 patients) were categorized as postmenopausal if >50 y old; otherwise, they were considered premenopausal.
Local regression (loess) was used to describe the relation between median consumption of selected food items (total vegetables, total cruciferous vegetables) and the year of admission (usually the same as the year at diagnosis). Loess is known as locally weighted polynomial regression proposed by Cleveland and colleagues (32, 33). As a nonparametric method, the loess curve often shows relatively complex relations and provides a graphic summary of the relation between a dependent variable and ≥1 independent variables (34). The median intake of each food group was calculated within each year, and the loess curves were produced to investigate the potential change in consumption pattern.
Two-tailed t tests and Pearson's chi-square tests were conducted to evaluate differences between cases and controls for continuous and categorical variables, respectively. ORs and 95% CIs for breast cancer in relation to cruciferous vegetable intake were calculated with unconditional logistic regression by using the lowest intake category as the referent. Potential variables as listed in Table 1 were evaluated by using stepwise selection approaches at the entry level of 0.1 and the stay level of 0.05. The significant covariates included family income (6 categories), BMI (continuous), age at menarche (continuous), age at first birth (continuous), parity (0, 1, 2, or ≥3 children), history of first-degree family member with breast cancer (yes or no), use of hormone replacement therapy (yes or no), and total meat intake (continuous). The final model also included cigarette smoking (never, former, or current smoker) due to different distributions between cases and controls in the study, as well as age (continuous) and year of admission (continuous). Age and year of admission were included to account for the potential change in diet habit and breast cancer diagnosis criteria over time. Multiplicative interactions were tested through the inclusion of cross-product terms in the logistic regression models. Statistical analyses and figure plotting were performed by using SAS for Windows, version 9.2. All tests were 2-sided and considered significant when P < 0.05.
TABLE 1.
Descriptive characteristics of breast cancer cases and hospital controls1
| All | Premenopausal women | Postmenopausal women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 1491) | Controls (n = 1482) | P | Cases (n = 394) | Controls (n = 428) | P | Cases (n = 1097) | Controls (n = 1054) | P | |
| Age, n (%) | 0.9994 | 0.9205 | 0.5048 | ||||||
| <46 y | 393 (26.4) | 392 (26.5) | 284 (72.1) | 307 (71.7) | 109 (9.9) | 85 (8.1) | |||
| 46–50 y | 183 (12.3) | 183 (12.3) | 84 (21.3) | 88 (20.6) | 99 (9.0) | 95 (9.0) | |||
| 51–55 y | 184 (12.3) | 184 (12.4) | 22 (5.6) | 27 (6.3) | 162 (14.8) | 157 (14.9) | |||
| >55 y | 731 (49.0) | 723 (48.8) | 4 (1.0) | 6 (1.4) | 727 (66.3) | 717 (68.0) | |||
| Year of admission, n (%) | 0.9745 | 0.5496 | 0.8078 | ||||||
| 1982–1985 | 492 (33.0) | 492 (33.2) | 124 (31.5) | 127 (29.7) | 368 (33.5) | 365 (34.6) | |||
| 1986–1990 | 421 (28.2) | 421 (28.4) | 114 (28.9) | 116 (27.1) | 307 (28.0) | 305 (28.9) | |||
| 1991–1995 | 409 (27.4) | 409 (27.6) | 107 (27.2) | 136 (31.8) | 302 (27.5) | 273 (25.9) | |||
| 1996–1998 | 169 (11.3) | 160 (10.8) | 49 (12.4) | 49 (11.4) | 120 (10.9) | 111 (10.5) | |||
| Education, n (%) | 0.8601 | 0.1254 | 0.9350 | ||||||
| Up to high school | 286 (19.3) | 287 (19.5) | 25 (6.4) | 33 (7.7) | 261 (23.9) | 254 (24.2) | |||
| High school graduate | 491 (33.1) | 502 (34.0) | 114 (29.0) | 138 (32.3) | 377 (34.6) | 364 (34.7) | |||
| Some college | 328 (22.1) | 329 (22.3) | 87 (22.1) | 109 (25.5) | 241 (22.1) | 220 (21.0) | |||
| College graduate | 379 (25.5) | 357 (24.2) | 167 (42.5) | 147 (34.4) | 212 (19.4) | 210 (20.0) | |||
| Ever had a job, n (%) | 0.8300 | 0.3260 | 0.8469 | ||||||
| Yes | 1427 (95.7) | 1416 (95.6) | 384 (97.5) | 412 (96.3) | 1043 (95.1) | 1004 (95.3) | |||
| No | 64 (4.3) | 66 (4.5) | 10 (2.5) | 16 (3.7) | 54 (4.9) | 50 (4.7) | |||
| Family income, n (%) | <0.0001 | <0.0001 | 0.0042 | ||||||
| <$25,000 | 843 (58.0) | 979 (67.5) | 137 (35.4) | 238 (56.4) | 706 (66.2) | 741 (72.0) | |||
| ≥$25,000 | 610 (42.0) | 472 (32.5) | 250 (64.6) | 184 (43.6) | 360 (33.8) | 288 (28.0) | |||
| BMI (in kg/m2), n (%) | 0.0041 | 0.6316 | 0.0056 | ||||||
| ≤24.9 | 751 (51.1) | 830 (56.8) | 245 (62.3) | 277 (65.5) | 506 (47) | 553 (53.3) | |||
| 25.0–29.9 | 428 (29.1) | 395 (27.0) | 91 (23.2) | 88 (20.8) | 337 (31.3) | 307 (29.6) | |||
| ≥30.0 | 291 (19.8) | 236 (16.2) | 57 (14.5) | 58 (13.7) | 234 (21.7) | 178 (17.1) | |||
| Cigarette smoking, n (%) | 0.0145 | 0.0387 | 0.1848 | ||||||
| Never | 770 (51.7) | 726 (49.1) | 204 (51.8) | 192 (45.0) | 566 (51.7) | 534 (50.8) | |||
| Former | 472 (31.7) | 447 (30.2) | 117 (29.7) | 126 (29.5) | 355 (32.4) | 321 (30.5) | |||
| Current | 246 (16.5) | 306 (20.7) | 73 (18.5) | 109 (25.5) | 173 (15.8) | 197 (18.7) | |||
| Alcohol intake, n (%) | 0.2319 | 0.4680 | 0.3351 | ||||||
| Never | 472 (32.8) | 513 (36.1) | 102 (26.6) | 127 (31.1) | 370 (35.1) | 386 (38.2) | |||
| 0 to <1 drinks/d | 745 (51.8) | 707 (49.8) | 223 (58.1) | 217 (53.1) | 522 (49.6) | 490 (48.5) | |||
| 1 to <2 drinks/d | 157 (10.9) | 135 (9.5) | 44 (11.5) | 46 (11.2) | 113 (10.7) | 89 (8.8) | |||
| ≥2 drinks/d | 63 (4.4) | 65 (4.6) | 15 (3.9) | 19 (4.6) | 48 (4.6) | 46 (4.5) | |||
| Age at menarche, n (%) | 0.0448 | 0.0274 | 0.1881 | ||||||
| <12 y | 295 (20.1) | 295 (20.3) | 70 (18.0) | 94 (22.4) | 225 (20.9) | 201 (19.5) | |||
| 12–13 y | 820 (55.9) | 753 (51.9) | 244 (62.9) | 225 (53.6) | 576 (53.4) | 528 (51.2) | |||
| ≥14 y | 352 (24.0) | 403 (27.8) | 74 (19.1) | 101 (24.0) | 278 (25.8) | 302 (29.3) | |||
| Parity, n (%) | 0.1745 | 0.3137 | 0.1179 | ||||||
| No children | 239 (16.1) | 216 (14.7) | 86 (21.8) | 79 (18.7) | 153 (14.0) | 137 (13.1) | |||
| 1 child | 188 (12.6) | 167 (11.4) | 70 (17.8) | 62 (14.7) | 118 (10.8) | 105 (10.0) | |||
| 2 children | 392 (26.3) | 366 (24.9) | 106 (26.9) | 129 (30.5) | 286 (26.1) | 237 (22.7) | |||
| ≥3 children | 669 (45.0) | 719 (49.0) | 132 (33.5) | 153 (36.2) | 537 (49.1) | 566 (54.2) | |||
| Age at first birth among parous women, n (%) | 0.0045 | 0.0480 | 0.0291 | ||||||
| <25 y | 746 (60.1) | 829 (66.7) | 189 (61.4) | 237 (69.3) | 557 (59.7) | 592 (65.7) | |||
| 25–29 y | 346 (27.9) | 290 (23.3) | 91 (29.5) | 69 (20.2) | 255 (27.3) | 221 (24.5) | |||
| 30–34 y | 107 (8.6) | 97 (7.8) | 22 (7.1) | 30 (8.8) | 85 (9.1) | 67 (7.4) | |||
| ≥35 y | 42 (3.4) | 27 (2.2) | 6 (1.9) | 6 (1.8) | 36 (3.9) | 21 (2.3) | |||
| Breastfeeding duration among parous women, n (%) | 0.7008 | 0.3701 | 0.1548 | ||||||
| <6 mo | 799 (67.7) | 807 (67.6) | 197 (68.2) | 217 (64.4) | 602 (67.5) | 590 (68.9) | |||
| 6 to <12 mo | 145 (12.3) | 158 (13.2) | 32 (11.1) | 34 (10.1) | 113 (12.7) | 124 (14.5) | |||
| ≥12 mo | 237 (20.1) | 228 (19.1) | 60 (20.8) | 86 (25.5) | 177 (19.8) | 142 (16.6) | |||
| History of benign breast disease, n (%) | 0.4466 | 0.3401 | 0.6916 | ||||||
| No | 908 (62.1) | 926 (63.5) | 218 (56.9) | 253 (60.2) | 690 (63.9) | 673 (64.8) | |||
| Yes | 554 (37.9) | 533 (36.5) | 165 (43.1) | 167 (39.8) | 389 (36.1) | 366 (35.2) | |||
| First-degree family member with breast cancer, n (%) | 0.0055 | 0.1155 | 0.0227 | ||||||
| No | 1235 (84.1) | 1270 (87.7) | 331 (84.7) | 367 (88.4) | 904 (83.9) | 903 (87.4) | |||
| Yes | 233 (15.9) | 178 (12.3) | 60 (15.3) | 48 (11.6) | 173 (16.1) | 130 (12.6) | |||
| Hormone replacement use n (%) | 0.0068 | 0.8217 | 0.0007 | ||||||
| No | 1186 (79.8) | 1110 (75.6) | 373 (95.2) | 402 (95.5) | 813 (74.2) | 708 (67.6) | |||
| Yes | 301 (20.2) | 358 (24.4) | 19 (4.8) | 19 (4.5) | 282 (25.8) | 339 (32.4) | |||
| Oral contraceptive use n (%) | 0.5067 | 0.3215 | 0.5577 | ||||||
| No | 929 (63.4) | 901 (62.2) | 121 (31.1) | 117 (27.9) | 808 (75.1) | 784 (76.2) | |||
| Yes | 536 (36.6) | 547 (37.8) | 268 (68.9) | 302 (72.1) | 268 (24.9) | 245 (23.8) | |||
Chi-square test was used to test the difference between cases and controls for categorical variables, and 2-tailed t test was used to test the difference between cases and controls for continuous variables.
Results
The descriptive characteristics of cases and controls are summarized in Table 1. No significant differences were observed between cases and controls for age at diagnosis and year of admission, indicating the successful matching on these 2 criteria. Compared with controls, cases tended to have a higher family income (P < 0.0001), a higher BMI (P = 0.0041), a younger age at menarche (P = 0.0448), an older age at first birth (P = 0.0045), and a greater likelihood of having a first-degree relative with breast cancer (P = 0.0055). Although the use of hormone replacement therapy was slightly lower in cases than in controls (20.2% compared with 24.4%), particularly among postmenopausal women (25.8% compared with 32.4%), hormone replacement use was a risk factor for breast cancer risk in our study with an OR of 1.27 (95% CI: 1.07, 1.51). There were more current smokers in the control group than in the case group; however, there was no significant difference in pack-years smoked between cases and controls (data not shown). In terms of education, employment, alcohol intake, parity, breastfeeding duration, history of benign breast disease, and oral contraceptive use, there were no significant differences between cases and controls.
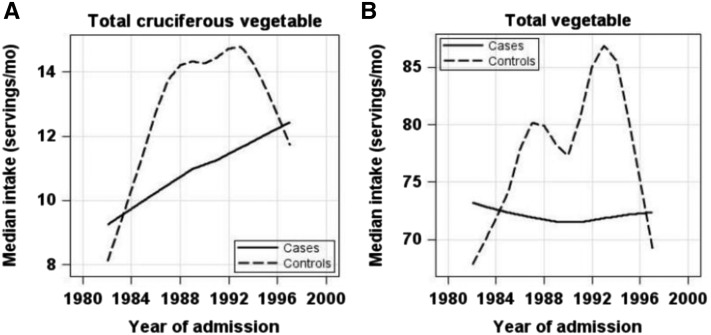
The median intakes of total cruciferous vegetables, vegetables, fruit, and meats were calculated within each year to examine the consumption patterns during 1982–1998 with the use of loess curves. As shown in Figure 1A, total cruciferous vegetable intake in controls increased after 1982, reached a plateau between 1988 and 1993, and then decreased after 1993. However, the intake of total cruciferous vegetables in cases showed a slow but steady increase from 1982 to 1998, although the magnitude of increase was smaller than that in controls. Interestingly, similar “increasing then declining” patterns were observed for total vegetable intake in controls, whereas cases showed no change in terms of total vegetable intake (Figure 1B).
FIGURE 1.
Changes in consumption of selected food items during 1982–1998 in breast cancer cases and controls. Loess curves were produced to examine the changes across the year of admission (usually the same as the year at diagnosis, x axis) in the median consumption of selected food items using the unit of servings per month (y axis): total cruciferous vegetables (A) and total vegetables (B). In each panel, the loess curves are presented as solid lines for cases and dashed lines for controls.
On the basis of the distribution of consumption in controls during 1982–1998, adjusted ORs and corresponding 95% CIs for the intake of cruciferous vegetables and breast cancer risk were examined separately by different year strata (Table 2). No significant association was observed in the “1982–1987” stratum, whereas a strong inverse association appeared in the strata of “1988–1992” and “1993–1998,” showing a significant dose-response relation within both strata. The inverse association tended to be more pronounced within more recent-year stratum, showing smaller ORs and narrower 95% CIs. For example, comparing the lowest to the highest intake category, ORs (95% CIs) for cruciferous vegetable intake and breast cancer risk changed from 0.89 (0.64, 1.23) in 1982–1987, to 0.58 (0.37, 0.92) in 1988–1992, and to 0.52 (0.33, 0.83) in 1993–1998. Similar trends were observed for total vegetable intake, whereas no significant association was observed for total fruit intake in any of the time periods (data not shown).
TABLE 2.
ORs (95% CIs) for the association of breast cancer with cruciferous vegetable consumption by strata of questionnaire completion year1
| 1982–1987 | 1988–1992 | 1993–1998 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 720) | Controls (n = 681) | OR (95% CI) | Cases (n = 313) | Controls (n = 420) | OR (95% CI) | Cases (n = 458) | Controls (n = 381) | OR (95% CI) | |
| Servings/mo | 15.5 ± 15.4 | 16.6 ± 17.1 | — | 17.5 ± 18.7 | 19.8 ± 17.0 | — | 18.0 ± 18.9 | 18.6 ± 17.2 | — |
| Q1 | 166 (23.1) | 168 (24.7) | 1.00 | 89 (28.4) | 103 (24.5) | 1.00 | 117 (25.5) | 84 (22.1) | 1.00 |
| Q2 | 223 (30.9) | 171 (25.1) | 1.23 (0.90, 1.67) | 84 (26.8) | 105 (25.0) | 0.83 (0.54, 1.28) | 135 (29.5) | 103 (27.0) | 0.82 (0.53, 1.27) |
| Q3 | 166 (23.1) | 171 (25.1) | 0.91 (0.66, 1.26) | 81 (25.9) | 105 (25.0) | 0.76 (0.49, 1.18) | 102 (22.3) | 98 (25.7) | 0.56 (0.35, 0.88) |
| Q4 | 165 (22.9) | 171 (25.1) | 0.89 (0.64, 1.23) | 59 (18.9) | 107 (25.5) | 0.58 (0.37, 0.92) | 104 (22.7) | 96 (25.2) | 0.52 (0.33, 0.83) |
| P-trend | 0.1389 | 0.0230 | 0.0037 | ||||||
Values are means ± SDs or n (%), unless otherwise indicated. ORs (95% CIs) were adjusted for age (continuous), year of admission (continuous), family income (6 categories), BMI (continuous), smoking status (current, former, or never), age at menarche (continuous), parity (no children, 1 child, 2 children, or ≥3 children), age at first birth (continuous), history of first-degree family member with breast cancer (yes or no), total meat intake (continuous), and hormone replacement use (yes or no). Q, quartile.
Combining all of the time periods, cruciferous vegetable intake was inversely associated with breast cancer risk, with a significant dose-response relation (P-trend = 0.0006) (Table 3). Adjustment of other vegetable intake in the analyses barely altered the associations (data not shown). Compared with those with the lowest intake, the highest intake was associated with a 32% reduction in odds of breast cancer (adjusted OR: 0.68; 95% CI: 0.55, 0.86). Similar significant inverse associations and dose-response relations were observed for broccoli and cauliflower intakes. Intakes of cabbage, Brussels sprouts, kale, and other greens also showed inverse associations with breast cancer risk, although the associations were not significant.
TABLE 3.
ORs (95% CIs) for the association of breast cancer with consumption of cruciferous vegetables
| n (%) | OR (95% CI) | |||
|---|---|---|---|---|
| Intake, servings/mo | Cases (n = 1491) | Controls (n = 1482) | Crude | Adjusted1 |
| Cruciferous vegetables | ||||
| <6 | 375 (25.2) | 341 (23.0) | 1.00 | 1.00 |
| 6–11.9 | 416 (27.9) | 389 (26.2) | 0.97 (0.79, 1.19) | 0.91 (0.74, 1.13) |
| 12–25.4 | 375 (25.2) | 373 (25.2) | 0.91 (0.74, 1.12) | 0.80 (0.64, 0.99) |
| ≥25.5 | 325 (21.8) | 379 (25.6) | 0.78 (0.63, 0.96) | 0.68 (0.55, 0.86) |
| P-trend | 0.0102 | 0.0006 | ||
| Broccoli | ||||
| <2.5 | 772 (51.8) | 695 (46.9) | 1.00 | 1.00 |
| 2.5–10 | 313 (21.0) | 319 (21.5) | 0.88 (0.73, 1.06) | 0.87 (0.72, 1.07) |
| ≥10 | 406 (27.2) | 468 (31.6) | 0.78 (0.66, 0.92) | 0.68 (0.56, 0.82) |
| P-trend | 0.0053 | <0.0001 | ||
| Cabbage | ||||
| <1 | 668 (44.8) | 659 (44.5) | 1.00 | 1.00 |
| 1–2.5 | 392 (26.3) | 355 (24.0) | 1.09 (0.91, 1.30) | 1.07 (0.88, 1.30) |
| 2.5 | 431 (28.9) | 468 (31.6) | 0.91 (0.77, 1.08) | 0.89 (0.74, 1.07) |
| P-trend | 0.3367 | 0.2687 | ||
| Cauliflower | ||||
| <1 | 562 (37.7) | 554 (37.4) | 1.00 | 1.00 |
| 1–4 | 648 (43.5) | 598 (40.4) | 1.07 (0.91, 1.26) | 0.99 (0.84, 1.18) |
| ≥4 | 281 (18.8) | 330 (22.3) | 0.84 (0.69, 1.02) | 0.76 (0.62, 0.94) |
| P-trend | 0.0367 | 0.0068 | ||
| Brussels sprouts | ||||
| <1 | 1085 (72.8) | 1062 (71.7) | 1.00 | 1.00 |
| ≥1 | 406 (27.2) | 420 (28.3) | 0.95 (0.81, 1.11) | 0.94 (0.79, 1.11) |
| Greens and kale | ||||
| <1 | 1224 (82.1) | 1187 (80.1) | 1.00 | 1.00 |
| ≥1 | 267 (17.9) | 295 (19.9) | 0.88 (0.73, 1.05) | 0.86 (0.70, 1.04) |
Adjusted for age (continuous), year of admission (continuous), family income (6 categories), BMI (continuous), smoking status (current, former, or never), age at menarche (continuous), parity (no children, 1 child, 2 children, or ≥3 children), age at first birth (continuous), history of first-degree family member with breast cancer (yes or no), total meat intake (continuous), and hormone replacement use (yes or no).
The association of cruciferous vegetable intake was further examined in raw and cooked forms separately with breast cancer risk in Table 4. For broccoli intake, a significant inverse association was observed for both raw (OR: 0.78; 95% CI: 0.66, 0.91) and cooked (OR: 0.83; 95% CI: 0.70, 0.99) intakes, whereas for cauliflower intake, raw consumption (OR: 0.77; 95% CI: 0.65, 0.90) but not cooked consumption (OR, 0.93; 95% CI: 0.79, 1.08) was significantly associated with breast cancer risk. The association with cabbage intake and breast cancer risk was much weaker, with a significant association for cooked cabbage consumption (OR: 0.84; 95% CI: 0.71, 0.99). In general, cruciferous vegetables consumed raw showed more pronounced inverse associations with breast cancer risk than did their cooked counterparts.
TABLE 4.
ORs (95% CIs) for the association of breast cancer with individual raw and cooked cruciferous vegetables
| n (%) | OR (95% CI) | |||
|---|---|---|---|---|
| Intake, servings/mo | Cases (n = 1491) | Controls (n = 1482) | Crude | Adjusted1 |
| Broccoli | ||||
| Raw | ||||
| <1 | 892 (59.8) | 813 (54.9) | 1.00 | 1.00 |
| ≥1 | 599 (40.2) | 669 (45.1) | 0.82 (0.71, 0.94) | 0.78 (0.66, 0.91) |
| Cooked | ||||
| <1 | 413 (27.7) | 383 (25.8) | 1.00 | 1.00 |
| ≥1 | 1078 (72.3) | 1099 (74.2) | 0.91 (0.77, 1.07) | 0.83 (0.70, 0.99) |
| Cabbage | ||||
| Raw | ||||
| <1 | 775 (52.0) | 791 (53.4) | 1.00 | 1.00 |
| ≥1 | 716 (48.0) | 691 (46.6) | 1.06 (0.92, 1.22) | 1.04 (0.89, 1.21) |
| Cooked | ||||
| <1 | 1017 (68.2) | 951 (64.2) | 1.00 | 1.00 |
| ≥1 | 474 (31.8) | 531 (35.8) | 0.83 (0.72, 0.97) | 0.84 (0.71, 0.99) |
| Cauliflower | ||||
| Raw | ||||
| <1 | 965 (64.7) | 894 (60.3) | 1.00 | 1.00 |
| ≥1 | 526 (35.3) | 588 (39.7) | 0.83 (0.71, 0.96) | 0.77 (0.65, 0.90) |
| Cooked | ||||
| <1 | 638 (42.8) | 632 (42.6) | 1.00 | 1.00 |
| ≥1 | 853 (57.2) | 850 (57.4) | 0.99 (0.86, 1.15) | 0.93 (0.79, 1.08) |
Adjusted for age (continuous), year of admission (continuous), family income (6 categories), BMI (continuous), smoking status (current, former, or never), age at menarche (continuous), parity (no children, 1 child, 2 children, or ≥3 children), age at first birth (continuous), history of first-degree family member with breast cancer (yes or no), total meat intake (continuous), and hormone replacement use (yes or no).
Associations between cruciferous vegetable intake and breast cancer risk were also investigated separately in pre- and postmenopausal women (Table 5). Significant inverse associations were observed only in premenopausal women (P-interaction = 0.0601) in a dose-dependent manner (P-trend = 0.0002). The same trend was found for individual cruciferous vegetables. For example, both raw and cooked broccoli intakes were significantly associated with reduced breast cancer risk (raw—OR: 0.59; 95% CI: 0.43, 0.80; cooked—OR: 0.49; 95% CI: 0.34, 0.70) in premenopausal women, whereas the associations in postmenopausal women were much weaker and became nonsignificant for either raw (OR: 0.87; 95% CI: 0.71, 1.05) or cooked (OR: 0.99; 95% CI: 0.80, 1.22) broccoli intake (P-interaction = 0.0397 and 0.0029 for raw and cooked broccoli intakes, respectively).
TABLE 5.
ORs (95% CIs) for the association of breast cancer with cruciferous vegetable intake among pre- and postmenopausal women
| Premenopausal women | Postmenopausal women | ||||||
|---|---|---|---|---|---|---|---|
| Intake, servings/mo | Cases (n = 394), n (%) | Controls (n = 428), n (%) | OR (95% CI)1 | Cases (n = 1097), n (%) | Controls (n = 1054), n (%) | OR (95% CI)1 | P-interaction |
| Cruciferous vegetables | 0.0601 | ||||||
| <6 | 122 (31.0) | 96 (22.4) | 1.00 | 253 (23.1) | 245 (23.2) | 1.00 | |
| 6 to <12 | 96 (24.4) | 107 (25.0) | 0.60 (0.40, 0.92) | 320 (29.2) | 282 (26.8) | 1.05 (0.82, 1.36) | |
| 12 to <25.5 | 98 (24.9) | 107 (25.0) | 0.57 (0.38, 0.87) | 277 (25.3) | 266 (25.2) | 0.90 (0.69, 1.18) | |
| ≥25.5 | 78 (19.8) | 118 (27.6) | 0.40 (0.26, 0.62) | 247 (22.5) | 261 (24.8) | 0.84 (0.64, 1.10) | |
| P-trend | 0.0002 | 0.0915 | |||||
| Broccoli | |||||||
| Raw | 0.0397 | ||||||
| <1 | 224 (56.9) | 196 (45.8) | 1.00 | 668 (60.9) | 617 (58.5) | 1.00 | |
| ≥1 | 170 (43.1) | 232 (54.2) | 0.59 (0.43, 0.80) | 429 (39.1) | 437 (41.5) | 0.87 (0.71, 1.05) | |
| Cooked | 0.0029 | ||||||
| <1 | 117 (29.7) | 87 (20.3) | 1.00 | 296 (27.0) | 296 (28.1) | 1.00 | |
| ≥1 | 277 (70.3) | 341 (79.7) | 0.49 (0.34, 0.70) | 801 (73.0) | 758 (71.9) | 0.99 (0.80, 1.22) | |
| Cabbage | |||||||
| Raw | 0.0470 | ||||||
| <1 | 263 (66.8) | 262 (61.2) | 1.00 | 512 (46.7) | 529 (50.2) | 1.00 | |
| ≥1 | 131 (33.2) | 166 (38.8) | 0.81 (0.59, 1.10) | 585 (53.3) | 525 (49.8) | 1.14 (0.95, 1.37) | |
| Cooked | 0.2115 | ||||||
| <1 | 309 (78.4) | 302 (70.6) | 1.00 | 708 (64.5) | 649 (61.6) | 1.00 | |
| ≥1 | 85 (21.6) | 126 (29.4) | 0.71 (0.50, 1.00) | 389 (35.5) | 405 (38.4) | 0.89 (0.73, 1.07) | |
| Cauliflower | |||||||
| Raw | 0.1343 | ||||||
| <1 | 249 (63.2) | 235 (54.9) | 1.00 | 716 (65.3) | 659 (62.5) | 1.00 | |
| ≥1 | 145 (36.8) | 193 (45.1) | 0.60 (0.44, 0.82) | 381 (34.7) | 395 (37.5) | 0.83 (0.69, 1.01) | |
| Cooked | 0.0115 | ||||||
| <1 | 196 (49.7) | 183 (42.8) | 1.00 | 442 (40.3) | 449 (42.6) | 1.00 | |
| ≥1 | 198 (50.3) | 245 (57.2) | 0.65 (0.48, 0.88) | 655 (59.7) | 605 (57.4) | 1.06 (0.88, 1.27) | |
| Brussels sprouts | 0.2911 | ||||||
| <1 | 290 (73.6) | 299 (69.9) | 1.00 | 795 (72.5) | 763 (72.4) | 1.00 | |
| ≥1 | 104 (26.4) | 129 (30.1) | 0.83 (0.60, 1.16) | 302 (27.5) | 291 (27.6) | 0.99 (0.81, 1.22) | |
| Greens and kale | 0.9453 | ||||||
| <1 | 336 (85.3) | 355 (82.9) | 1.00 | 888 (80.9) | 832 (78.9) | 1.00 | |
| ≥1 | 58 (14.7) | 73 (17.1) | 0.85 (0.56, 1.29) | 209 (19.1) | 222 (21.1) | 0.86 (0.68, 1.07) | |
ORs (95% CIs) were adjusted for age (continuous), year of admission (continuous), family income (6 categories), BMI (continuous), smoking status (current, former, or never), age at menarche (continuous), parity (no children, 1 child, 2 children, or ≥3 children), age at first birth (continuous), history of first-degree family member with breast cancer (yes or no), total meat intake (continuous), and hormone replacement use (yes or no).
Discussion
In this hospital-based case-control study, we found that the consumption of cruciferous vegetables increased during 1982–1998, but patterns of increases differed between breast cancer cases and controls. We also observed inverse associations between cruciferous vegetable intake and breast cancer risk, and the associations became more pronounced within more recent-year strata, which is consistent with the general trend of increased consumption among controls. When further stratified by pre- and postmenopausal status, a significant association between cruciferous vegetable intake and breast cancer risk was observed in premenopausal women only. When individual cruciferous vegetables were examined, only broccoli and cauliflower intakes showed a significant inverse association with breast cancer risk; and consistently, the associations were observed only in premenopausal women and appeared to be stronger with raw vegetable consumption than with their cooked vegetable counterparts.
Cruciferous vegetable intake changed considerably during 1982–1998 among the controls. As loess curves show (Figure 1), the consumption of cruciferous vegetables in controls had 3 phases: median consumption increased from 9 to 16 servings/mo during 1982–1988, reached a plateau during 1988–1993 with a median of 15 servings/mo, and then declined from 1993 (Supplemental Table 1). Similar patterns were also observed for total vegetable intake in controls. Many factors may contribute to consumption pattern changes. Interestingly, these changes coincided with the timelines of our understanding of diet and cancer, including cruciferous vegetables. Since the beginning of the 1980s, mounting evidence has been published on vegetable intake and reduction in cancer risk, accompanying various diet-related campaigns for cancer prevention. One of the most important national campaigns in the United States was the 5 A Day program, which was initiated by the National Cancer Institute in 1991; later, the CDC became the lead federal agency and changed the program name to Fruits & Veggies—More Matters (35). The second wave of increasing vegetable intake from 1991 to 1993 in controls (Figure 1B) may be the reflection of this campaign. We further expanded the analysis to include all controls in the PEDS. Similar patterns were observed for all female controls (Supplemental Figure 1; n = 5940) as well as total male and female controls (Supplemental Figure 2; n = 8685), indicating that the observed consumption patterns in controls were not due to matching with breast cancer cases. On the basis of data from the USDA, the consumption of broccoli, the representative cruciferous vegetable, indeed increased over time, doubling per capita consumption during 1982–1998 (36). Cruciferous vegetable consumption in our study followed the same trend, although the data were derived from hospital-based controls instead of the general population. Note that the intakes of vegetables and cruciferous vegetables in controls all started to decline from 1993. It is not clear whether this decline was related to phases of public campaigns; however, this calls for continuity of and consistency in public education.
Interestingly, compared with the consumption changes in controls, breast cancer cases behaved differently. Cases did not show an increase in vegetable consumption as did controls. In fact, total vegetable intake remained flat during 1982–1998 in cases. For cruciferous vegetables, the consumption increased over time in both cases and controls, although the increase in cases was slower and smaller than that in controls (Figure 1A). The divergent consumption patterns between cruciferous vegetables and total vegetables among cases may indicate that specific food categories rather than general food items may be more attractive to the public in terms of cancer prevention. It should be noted that the consumption in the study is self-reported. Cases may have differential recall bias than controls, perhaps reporting higher intakes of cruciferous vegetables than their actual intakes. However, the consumption of either cruciferous vegetables or total vegetables was generally lower among cases than controls, indicating that there are still existing areas for continued public health education.
In response to changes in cruciferous vegetable consumption during 1982–1998, the associations with breast cancer risk also varied accordingly, showing strengthened associations in recent-year strata (Table 2). The more pronounced associations in recent years may be attributed to the increased variability in consumption due to different consumption patterns between cases and controls. It is noteworthy that the cutoff selection was based on the consumption in controls in each corresponding year stratum; therefore, the quartile levels were different across 3-y strata as shown by varied means for cases and controls among each year stratum (Table 2). These results indicate that association studies with food consumption may be affected by the time frame of the studies conducted, which may potentially contribute to the mixed results in the literature. Overall, a significant inverse association was observed between cruciferous vegetable intake and breast cancer risk. However, the potential effect of cruciferous vegetables on breast cancer is only limited to premenopausal women but not postmenopausal women (Table 5). This is unlikely to be a chance finding because each individual cruciferous vegetable also showed the same trend. Mounting evidence suggests that mechanisms may be different in breast cancer etiology in pre- and postmenopausal women (37–39). In addition to antiproliferative activity against breast cancer cells, dietary ITCs have been shown to modulate estrogen metabolism (16–19), which may have a greater impact on premenopausal women than on postmenopausal women.
It has been established that cooking processes could lead to heat-inactivation of myrosinase, the enzyme catalyzing the release of ITCs from the precursors in the vegetables, and the destruction of heat-labile ITCs (27–29). Few previous studies separately investigated raw compared with cooked cruciferous vegetable intake in relation to breast cancer risk, which could partly explain the inconsistency in the existing literature, because eating or cooking styles vary across different populations. In the current study, the 3 most commonly consumed cruciferous vegetables in the United States—broccoli, cauliflower, and cabbage—were examined separately in raw and cooked forms (Table 4). Both broccoli and cauliflower showed stronger inverse associations with breast cancer risk when consumed raw than when consumed cooked. However, a borderline significant association was observed with cooked cabbage intake but not with raw cabbage intake. It is possible that coleslaw consumption (counted as raw consumption) dilutes the association of raw cabbage intake with breast cancer risk, because it is usually consumed with mayonnaise, buttermilk, cream, etc., and high fat intake is a suspected risk factor for breast cancer (40). No significant associations were observed with intakes of Brussels sprouts, kale, and collard, mustard, and turnip greens, which may be partly related to the less common consumption and frequent overcooking of these vegetables.
Several limitations need to be discussed. Recall bias is always a concern in case-control studies. The FFQ was completed after diagnosis, and so may not provide a precise assessment of dietary habits before disease. However, in the current study, cases reported a steady increase in cruciferous vegetable intake during 1982–1998. If this phenomenon is due to overreporting, the inverse association between cruciferous vegetable intake and breast cancer risk may be underestimated in the current study. Selection bias may also occur. Both cases and controls were limited to individuals who came to the RPCI, a large regional comprehensive cancer center. However, the demographic characteristics of our population are comparable to the general population as shown in Table 1. Therefore, representativeness might not be a main issue. It needs to be pointed out that due to lack of physical activity information and to participants who were predominantly white (99%), these 2 important factors could not be considered in the study.
In summary, the current study suggests that cruciferous vegetable intake is associated with reduced breast cancer risk, in particular with broccoli and cauliflower intakes. The inverse association of cruciferous vegetable consumption with breast cancer risk seems to be more apparent in premenopausal women. Biologically, dietary ITCs obtained from cruciferous vegetables have been shown to modulate estrogen metabolism, which may, at least partly, explain the differential effect of cruciferous vegetable intake on pre- and postmenopausal women, although other mechanisms may apply. These data also indicate the successful impact of public health efforts on encouraging vegetable consumption, which may change consumption patterns and modify the results of association studies on vegetables and disease.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—TL, SEM, KBM, CBA, and LT: designed the research; TL, GRZ, and LT: performed the statistical analysis; TL, SEM, CBA, and LT: wrote the manuscript; LT: had primary responsibility for final content; and all authors: critically revised the manuscript and read and approved the final manuscript.
Abbreviations
- ITC
isothiocyanate
- PEDS
Patient Epidemiology Data System
- RPCI
Roswell Park Cancer Institute
Footnotes
Supported by the National Cancer Institute (K07CA148888).
References
- 1. American Cancer Society Breast cancer statistics [cited 2017 Jan 8]. Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics.
- 2. Fuentes F, Paredes-Gonzalez X, Kong AT.. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1:179–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujioka N, Fritz V, Upadhyaya P, Kassie F, Hecht SS.. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: a tribute to Lee W. Wattenberg. Mol Nutr Food Res 2016;60:1228–38. [DOI] [PubMed] [Google Scholar]
- 4. Higdon JV, Delage B, Williams DE, Dashwood RH.. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 2007;55:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Tang L.. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin 2007;28:1343–54. [DOI] [PubMed] [Google Scholar]
- 6. Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen M-SA, Stierer T, Garrett-Mayer E, Argani P.. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007;28:1485–90. [DOI] [PubMed] [Google Scholar]
- 7. Singletary K, MacDonald C.. Inhibition of benzo [a] pyrene-and 1, 6-dinitropyrene-DNA adduct formation in human mammary epithelial cells bydibenzoylmethane and sulforaphane. Cancer Lett 2000;155:47–54. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y.. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res 2004;555:173–90. [DOI] [PubMed] [Google Scholar]
- 9. Brandi G, Paiardini M, Cervasi B, Fiorucci C, Filippone P, De Marco C, Zaffaroni N, Magnani M.. A new indole-3-carbinol tetrameric derivative inhibits cyclin-dependent kinase 6 expression, and induces G1 cell cycle arrest in both estrogen-dependent and estrogen-independent breast cancer cell lines. Cancer Res 2003;63:4028–36. [PubMed] [Google Scholar]
- 10. Cover CM, Hsieh SJ, Cram EJ, Hong C, Riby JE, Bjeldanes LF, Firestone GL.. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res 1999;59:1244–51. [PubMed] [Google Scholar]
- 11. Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL.. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem 1998;273:3838–47. [DOI] [PubMed] [Google Scholar]
- 12. Rahman KM, Aranha O, Glazyrin A, Chinni SR, Sarkar FH.. Translocation of Bax to mitochondria induces apoptotic cell death in indole-3-carbinol (I3C) treated breast cancer cells. Oncogene 2000;19:5764–71. [DOI] [PubMed] [Google Scholar]
- 13. Tseng E, Scott-Ramsay EA, Morris ME.. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood) 2004;229:835–42. [DOI] [PubMed] [Google Scholar]
- 14. Warin R, Chambers WH, Potter DM, Singh SV.. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res 2009;69:9473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashok BT, Chen Y, Liu X, Bradlow HL, Mittelman A, Tiwari RK.. Abrogation of estrogen-mediated cellular and biochemical effects by indole-3-carbinol. Nutr Cancer 2001;41:180–7. [DOI] [PubMed] [Google Scholar]
- 16. Bradlow HL, Michnovicz JJ, Halper M, Miller DG, Wong G, Osborne MP.. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol Biomarkers Prev 1994;3:591–5. [PubMed] [Google Scholar]
- 17. Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF.. Pilot study: effect of 3, 3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer 2004;50:161–7. [DOI] [PubMed] [Google Scholar]
- 18. McAlindon TE, Gulin J, Chen T, Klug T, Lahita R, Nuite M.. Indole-3-carbinol in women with SLE: effect on estrogen metabolism and disease activity. Lupus 2001;10:779–83. [DOI] [PubMed] [Google Scholar]
- 19. Michnovicz JJ, Adlercreutz H, Bradlow HL.. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst 1997;89:718–23. [DOI] [PubMed] [Google Scholar]
- 20. Taketo MM.. Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J Natl Cancer Inst 1998;90:1529–36. [DOI] [PubMed] [Google Scholar]
- 21. Uto T, Hou DX, Morinaga O, Shoyama Y.. Molecular mechanisms underlying anti-inflammatory actions of 6-(methylsulfinyl) hexyl isothiocyanate derived from wasabi (Wasabia japonica). Adv Pharmacol Sci 2012;2012:614046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG.. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr 2004;134:1134–8. [DOI] [PubMed] [Google Scholar]
- 23. Gaudet MM, Britton JA, Kabat GC, Steck-Scott S, Eng SM, Teitelbaum SL, Terry MB, Neugut AI, Gammon MD.. Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev 2004;13:1485–94. [PubMed] [Google Scholar]
- 24. Zhang CX, Ho SC, Chen YM, Fu JH, Cheng SZ, Lin FY.. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer 2009;125:181–8. [DOI] [PubMed] [Google Scholar]
- 25. Shannon J, Ray R, Wu C, Nelson Z, Gao DL, Li W, Hu W, Lampe J, Horner N, Satia J, et al. Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2005;14:81–90. [PubMed] [Google Scholar]
- 26. Liu X, Lv K.. Cruciferous vegetables intake is inversely associated with risk of breast cancer: a meta-analysis. Breast 2013;22:309–13. [DOI] [PubMed] [Google Scholar]
- 27. Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung F-L.. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 2000;38:168–78. [DOI] [PubMed] [Google Scholar]
- 28. Getahun SM, Chung F-L.. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev 1999;8:447–51. [PubMed] [Google Scholar]
- 29. Rouzaud G, Young SA, Duncan AJ.. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol Biomarkers Prev 2004;13:125–31. [DOI] [PubMed] [Google Scholar]
- 30. Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA.. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev 1996;5:733–48. [PubMed] [Google Scholar]
- 31. Byers T, Marshall J, Fiedler R, Zielezny M, Graham S.. Assessing nutrient intake with an abbreviated dietary interview. Am J Epidemiol 1985;122:41–50. [DOI] [PubMed] [Google Scholar]
- 32. Cleveland WS.. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–36. [Google Scholar]
- 33. Cleveland WS, Devlin SJ.. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 1988;83:596–610. [Google Scholar]
- 34. Jacoby WG.. Loess: a nonparametric, graphical tool for depicting relationships between variables. Elect Stud 2000;19:577–613. [Google Scholar]
- 35. CDC 5 A day works! [Internet] [cited 2017 Jan 8]. Available from: http://www.cdc.gov/nccdphp/dnpa/nutrition/health_professionals/programs/5aday_works.pdf.
- 36. Economics USDA, Statistics and Market Information System US broccoli statistics [cited 2017 Jan 8]. Available from: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1816.
- 37. Hirose K, Tajima K, Hamajima N, Inoue M, Takezaki T, Kuroishi T, Yoshida M, Tokudome S.. A large-scale, hospital-based case-control study of risk factors of breast cancer according to menopausal status. Jpn J Cancer Res 1995;86:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reinier KS, Vacek PM, Geller BM.. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and post-menopausal women. Breast Cancer Res Treat 2007;103:343–8. [DOI] [PubMed] [Google Scholar]
- 39. Kruk J.. Association of lifestyle and other risk factors with breast cancer according to menopausal status: a case-control study in the region of Western Pomerania (Poland). Asian Pac J Cancer Prev 2007;8:513–24. [PubMed] [Google Scholar]
- 40. Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S.. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer 2003;89:1672–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.