Abstract
Background: Low protein intake is associated with various negative health outcomes at any life stage. When diets do not contain sufficient protein, phosphorus availability is compromised because proteins are the major sources of phosphorus. However, whether mineral phosphorus supplementation mitigates this problem is unknown, to our knowledge.
Objective: Our goal was to determine the impact of dietary phosphorus supplementation on food intake, weight gain, energy efficiency, body composition, blood metabolites, and liver histology in rats fed a low-protein diet for 9 wk.
Methods: Forty-nine 6-wk-old male Sprague-Dawley rats were randomly allocated to 5 groups and consumed 5 isocaloric diets ad libitum that varied only in protein (egg white) and phosphorus concentrations for 9 wk. The control group received a 20% protein diet with 0.3% P (NP-0.3P). The 4 other groups were fed a low-protein (10%) diet with a phosphorus concentration of 0.015%, 0.056%, 0.1%, or 0.3% (LP-0.3P). The rats' weight, body and liver composition, and plasma biomarkers were then assessed.
Results: The addition of phosphorus to the low-protein diet significantly increased food intake, weight gain, and energy efficiency, which were similar among the groups that received 0.3% P (LP-0.3P and NP-0.3P) regardless of dietary protein content. In addition, phosphorus supplementation of low-protein diets reduced plasma urea nitrogen and increased total body protein content (defatted). Changes in food intake and efficiency, body weight and composition, and plasma urea concentration were highly pronounced at a dietary phosphorus content <0.1%, which may represent a critical threshold.
Conclusions: The addition of phosphorus to low-protein diets improved growth measures in rats, mainly as a result of enhanced energy efficiency. A dietary phosphorus concentration of 0.3% mitigated detrimental effects of low-protein diets on growth parameters.
Keywords: low-protein diet, phosphorus, weight gain, food intake, energy efficiency, nonalcoholic fatty liver disease, Sprague-Dawley rats
Introduction
Protein deficiency can lead to various adverse health effects at any life stage. Protein restriction compromises growth and muscle maintenance in adolescents and leads to weight loss, reduced subcutaneous fat, increased susceptibility to infection, general lethargy, and delayed wound healing in adults (1). In addition, inadequate protein content in maternal diets during pregnancy is reported to result in negative health outcomes in offspring (2). Experiments in rodents have shown that a maternal low-protein diet during pregnancy is associated with disproportionate patterns of fetal growth (3), increased adult onset of metabolic disorders, and increased adiposity and glucose intolerance (4). Furthermore, low dietary protein intake has consistently been associated with increased body fat content in rodents (5–8).
Alterations in food intake (9, 10), organ size, and enzymatic function (11, 12) are believed to mediate animals' metabolic response to protein restriction. Changes in food intake were reported to be inconsistent; some studies showed an increase (7, 8, 13) and others reported a decrease that was dependent on the extent of protein restriction (10, 14). This inconsistency may imply that other factors are involved in the regulation of food intake.
Although proteins vary in phosphorus content and bioavailability (15), the synergistic relationship between dietary protein and phosphorus intake suggests that dietary phosphorus modulates the effects of protein restriction. Phosphorus is an essential mineral and thus plays an important role in cellular metabolism. It is also integrated within ATP, which acts as a phosphate donor for many metabolic reactions (16). Such reactions are strongly dependent on the body's phosphorus availability for its production (17, 18), and this is related to dietary phosphorus availability.
Populations whose staple foods are low in protein quantity and quality (e.g., cassava, maize, rice) are most at risk of developing diseases associated with protein malnutrition (e.g., marasmus and kwashiorkor) (19). Low dietary phosphorus results from low protein in the diet, which tends to compound nutritional problems. These populations also consume minimal amounts of animal and dairy products, which are important dietary sources of both high-quality proteins and phosphorus. In contrast, most stable plant-based foods that are also commonly consumed by at-risk populations (e.g., whole wheat or brown rice) have low protein quantity and quality and contain low amounts of bioavailable phosphorus because phosphorus is mainly bound in the form of phytate, which makes it unavailable for absorption (20). Fermentation is known to break down phytate and thus improves the bioavailability of phosphorus (21). Fermentation of yeast for ∼60 min was reported to reduce the phytate content of bread by ∼10% (22).
Phosphorus intake is compromised under conditions of protein restriction; therefore, it can be assumed that inadequate dietary phosphorus intake compounds the negative effects of protein restriction. Accordingly, this study was performed to assess the impact of phosphorus on food intake, weight gain, food efficiency, and body composition of rats maintained with a low-protein diet with varied concentrations of dietary phosphorus.
Methods
Animal housing
Forty-nine 6-wk-old male Sprague-Dawley rats (American University of Beirut Animal Care Facility) were housed individually in a room with controlled temperature (mean ± SD: 22 ± 1°C) and light (12-h light-dark cycle, with lights on at 0700). The rats had free access to water and consumed a semisynthetic powder control diet ad libitum (Supplemental Table 1) for 1 wk to familiarize them with the environment and the diet.
Experimental diet
The semisynthetic powder experimental diets (Supplemental Table 1) were isocaloric and were all prepared using the same ingredients. Dried egg white was used as the main source of protein because it supplies all essential amino acids and contains negligible amounts of phosphorus (mean ± SD: 1.5 ± 0.013 g/kg) (23). Phosphorus-free mineral mix (AIN-93G mix without phosphorus) and potassium phosphate from Dyets Inc. were used to modify the phosphorus content of the diet. Potassium phosphate was used because the addition of potassium does not affect growth (24, 25).
Experimental design
The experimental protocol was approved by the American University of Beirut Institutional Animal Care and Use Committee. After the 1-wk acclimation period, each rat (weight range: 190–315 g) was randomly allocated to 1 of 5 experimental groups. Under normal conditions, the recommended phosphorus content of the rats' diet is 0.3% (26), which was the dietary proportion used for the control group. Treatment groups were as follows: 20% protein and 0.3% P (NP-0.3P; control), 10% protein and 0.015% P (LP-0.015P), 10% protein and 0.056% P (LP-0.056P), 10% protein and 0.1% P (LP-0.1P), and 10% protein and 0.3% P (NP-0.3P) (Supplemental Table 1).
The rats were offered their corresponding diets ad libitum for 9 wk. At the study termination, overnight-fasted rats were anesthetized with isoflurane (Forane; Abbott) and blood was collected from the superior vena cava. The rats were then euthanized by removing the heart and their livers were extracted and weighed. Immediately afterward, 2 small liver sections were taken for histologic analysis and the remaining section was frozen in liquid nitrogen and stored at −80°C. Blood samples were centrifuged at 2200 × g for 15 min at 3°C and plasma aliquots were collected and stored at −80°C. The rat carcasses were stored at −20°C for body composition analysis.
Food intake and body weight and composition
Food intake and body weight were measured twice per week and then averaged to calculate weekly changes. Carcasses were dried until they reached a constant weight (∼48 h) at 105°C and homogenized, and fat was extracted using petroleum ether (boiling point: 40–60°C). The moisture and fat content of the carcass was calculated from the differences in weight. Hepatic fat content was determined as follows: ∼2 g of liver was freeze-dried (2.5-L benchtop freeze-dry system; Labconco) for 48 h and fat was extracted with petroleum ether solvent (boiling point: 40–60°C) for 40 min using an ANKOMXT10 extractor (ANKOM Technology).
Blood analysis
Fasting plasma glucose, TG, total cholesterol, and total phosphorus were determined with an enzymatic colorimetric method on the Vitros 350 Chemistry System (Johnson & Johnson Ortho-Clinical Diagnostics). The plasma insulin concentration was determined by an enzyme immunoassay using an insulin rat ELISA kit (EZRMI-13K; EMD Millipore Corporation).
Liver biopsy
Small liver sections were stained with hematoxylin and eosin (H&E) and Oil Red O (ORO) for evaluation of necroinflammatory grading and fatty droplets. Histologic changes were assessed by modifying the scoring system for grading and staging for nonalcoholic fatty liver disease as described by Kleiner et al. (27).
Histopathology examination
Rat liver tissue was processed into 3- to 4-µm-thick formalin-fixed paraffin-embedded tissue sections and stained with H&E. Histopathologic examination consisted of assessing a steatosis grade and distribution score of 0 (<5%), 1 (5–33%), 2 (33–66%), or 3 (>66%). Location was defined as a steatosis distribution score of 0 (zone 3), 1 (zone 1), 2 (azonal), or 3 (panacinar). Microvesicular steatosis was recorded as a score of either 0 (not present) or 1 (present). Lobular inflammation was semiquantified according to a score of 0 (<2 foci per ×200 field), 1 (2–4 foci per ×200 field), or 3 (>4 foci per ×200 field).
ORO examination
ORO staining was performed according to a previously described protocol (28). Briefly, fresh-frozen rat liver tissue was embedded into cryomolds and sectioned into 5-µm sections on a cryostat (Leica). Sections were then stained with ORO and were semiquantified with NIH ImageJ software (http://rsbweb.nih.gov/ij). Tissue sections were imaged at 5 high-power (×400) fields and converted to 8-bit grayscale images. This was compared against a predefined image threshold according to a rat liver section negative for steatosis and microvesicular steatosis on H&E and ORO staining, and image analysis for ORO surface area staining determined.
Statistical analysis
The required number of rats (n = 9) was based on previous weight gain data (6.0 ± 0.95 g/d), assuming a 25% difference in the mean with 90% statistical power and 5% significance. Data are expressed as means ± SDs for all values. Data analysis was performed using SPSS 23 software (IBM SPSS). Results were analyzed by one-factor ANOVA, and specific comparisons were made between each of the 5 groups using Fisher's pairwise comparisons. P < 0.05 was considered significant.
Results
Weight gain, food intake, and energy efficiency
Although initial body weights were similar among the groups, the final body weights were significantly different. The final body weight of the control group (NP-0.3P) was significantly greater than that of the LP-0.015P and LP-0.056P groups. Among the low-protein groups, body weight increased with an increased dietary phosphorus content and the body weight of the LP-0.1P and LP-0.3P groups was not significantly different from that of the NP-0.3P group (Table 1).
TABLE 1.
Body weight and body composition of rats fed a control diet or 1 of 4 low-protein diets with different phosphorus concentrations for 9 wk1
| Variable | LP-0.015P (n = 9) | LP-0.056P (n = 10) | LP-0.1P (n = 10) | LP-0.3P (n = 10) | NP-0.3P (n = 10) | ANOVA P value |
|---|---|---|---|---|---|---|
| Initial body weight, g | 266.1 ± 35.7 | 265.4 ± 32.8 | 266.5 ± 27.6 | 267.8 ± 24.2 | 266.9 ± 31.9 | 1.000 |
| Final body measurements | ||||||
| Weight, g | 383 ± 42.6a | 426 ± 39.5a | 513 ± 50.6b | 536 ± 76.7b | 563 ± 60.6b | <0.001 |
| Water | ||||||
| g | 224.4 ± 25.6a | 237.1 ± 21.4a,c | 261.1 ± 22.4a,b | 253.5 ± 17.8b,c | 277.1 ± 19.5d | <0.001 |
| % | 65.4 ± 2.1a | 62.4 ± 3.7a,b | 57.2 ± 6.3b,c | 53.9 ± 9.7c | 55.1 ± 5.6c | <0.001 |
| Fat | ||||||
| g | 24.9 ± 9.4a | 33.8 ± 15.8a,b | 56.6 ± 32.1b,c | 64.9 ± 37.7c | 56.4 ± 21.2a,b | 0.006 |
| % | 20.6 ± 5.0 | 23.1 ± 6.1 | 27.3 ± 8.6 | 26.7 ± 8.5 | 24.2 ± 5.3 | 0.230 |
| Defatted | ||||||
| g | 94.5 ± 9.9a | 110.1 ± 14.9a | 142.7 ± 22.5b | 163.2 ± 46.7b,c | 174.0 ± 33.7c | <0.001 |
| % | 14.0 ± 3.7a | 14.5 ± 3.9a | 15.5 ± 5.6a,b | 19.5 ± 5.6b,c | 20.8 ± 5.4c | 0.010 |
| Liver | ||||||
| Weight | ||||||
| g | 12.0 ± 2.1a | 12.5 ± 1.9a | 15.3 ± 2.4b | 16.4 ± 3.3b | 16.3 ± 2.3b | <0.001 |
| g/100 g | 3.3 ± 0.4 | 3.1 ± 0.3 | 3.2 ± 0.4 | 3.2 ± 0.2 | 3.09 ± 0.2 | 0.545 |
| Water, % | 72.2 ± 1.5 | 71.1 ± 2.2 | 70.5 ± 1.6 | 70.7 ± 1.3 | 70.7 ± 1.0 | 0.187 |
| Fat, % | 8.0 ± 2.0 | 13.1 ± 5.9 | 11.0 ± 3.6 | 11.9 ± 6.5 | 11.0 ± 2.7 | 0.186 |
Values are means ± SDs. One-factor ANOVA was used to detect significant differences between groups. Significance was set at P < 0.05. Categories in the same row that do not share the same superscript letters are significantly different. LP-0.015P, 10% protein and 0.015% P; LP-0.056P, 10% protein and 0.056% P; LP-0.1P, 10% protein and 0.1% P; LP-0.3P, 10% protein and 0.3% P; NP-0.3P, 20% protein and 0.3% P (control group).
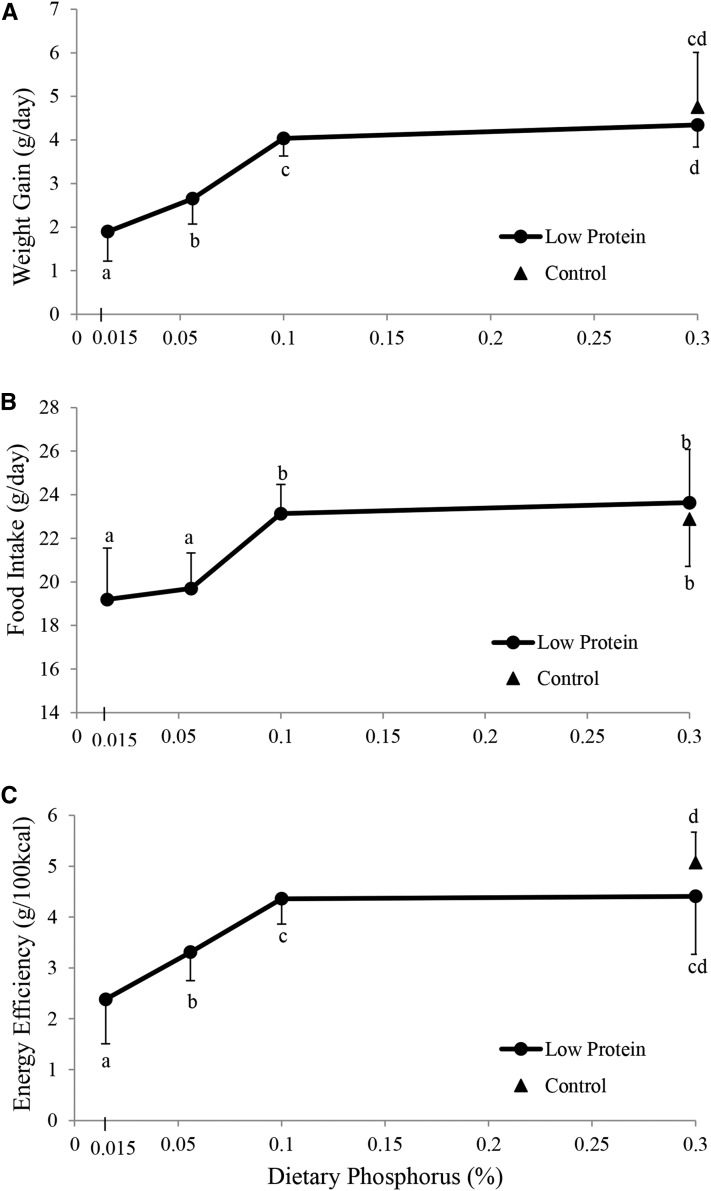
The addition of phosphorus to low-protein diets increased weight gain (expressed as g/d); weight gain in the LP-0.1P and LP-0.3P groups was close to that of the NP-0.3P group (. Moreover, food intake was improved by increasing the phosphorus content of the low-protein diets. No difference in food intake was observed among the LP-0.1P, LP-0.3P, and NP-0.3P groups (Figure 1B,Supplemental Figure 1). The difference in food intake of the low phosphorus (LP-0.015P and LP-0.056P) groups started with the introduction of the various diets and persisted throughout the experimental period (Supplemental Figure 1). The pattern of variation in energy efficiency [expressed as weight gain (g)/100 kcal] paralleled that of weight gain, in which energy efficiency of the low-protein groups increased with the addition of phosphorus to the diet, in which that of the LP-0.015P was lower than that of LP-0.056P that was lower than that of LP-0.1P. However, no differences were observed between energy efficiency of the LP-0.1P and LP-0.3P groups that was similar to that of the NP-0.3P group (Figure 1C).
FIGURE 1.
Mean daily weight gain (A), food intake (B), and energy efficiency (C) of rats fed a control diet or 1 of 4 low-protein diets with different phosphorus concentrations for 9 wk. Triangles indicate the control group (20% protein and 0.3% P). Circles indicate the 4 low-protein groups (10% protein with 0.015% P, 0.056% P, 0.1% P, or 0.3% P). Food intake, weight gain, and energy efficiency are expressed as means ± SDs as a function of the level of phosphorus in the diet (n = 9 or 10). Energy efficiency was calculated as the mean weight gained per day per 100 kcal of the corresponding diet consumed. One-factor ANOVA and specific comparisons were made between each of the 5 groups using Fisher's pairwise comparisons. Values that do not share the same superscript letter are significantly different (P < 0.05).
Body and liver composition
Results of the body proximate analysis suggested that the addition of phosphorus to low-protein diets was associated with an increase in body moisture. However, the proportion of body moisture decreased with the addition of phosphorus to low-protein diets. The proportion of moisture in the NP-0.3P group was similar to that of the LP-0.3P group and both were significantly less than the proportion of moisture in LP-0.015P– and LP-0.056P–fed rats. The addition of phosphorus to low-protein diets was associated with a significant increase in total body fat content (P < 0.01), but no significant changes in percentage of body fat were observed (P = 0.23). The defatted carcass weight was used to provide information on body protein status, because mineral content is known to be relatively small. The quantity and percentage of defatted rat carcasses in the LP-0.3P and NP-0.3P treatment groups was similar regardless of dietary protein content and was significantly greater than that of the LP-0.015P and LP-0.056P groups (Table 1).
In the low-protein groups, liver weight increased with increasing dietary phosphorus but liver weights of LP-0.1P and LP-0.3P rats were similar to NP-0.3P rats. However, when liver weight was expressed per 100 g of body weight, no statistically significant differences were detected among treatment groups (P = 0.55). In addition, no significant differences in percentages of hepatic dry weight or fat content were observed among the groups (Table 1).
Plasma results
The total plasma phosphorus concentration was significantly different among groups (P < 0.01); the LP-0.015P treatment group had the lowest value, whereas the other treatment groups maintained similar levels. The plasma glucose concentration in the NP-0.3P group was similar to the LP-0.3P group. Among the low-protein groups, the plasma glucose concentration increased with the increased phosphorus content of the diet. Plasma TG, total cholesterol, albumin, and C-reactive protein concentrations were not significantly different among treatment groups and were not affected by either the protein or phosphorus content of the diets (Table 2).
TABLE 2.
Plasma metabolites of rats fed a control diet or 1 of 4 low-protein diets with different phosphorus concentrations for 9 wk1
| Variable | LP-0.015P (n = 9) | LP-0.056P (n = 10) | LP-0.1P (n = 10) | LP-0.3P (n = 10) | NP-0.3P (n = 10) | ANOVA P value |
|---|---|---|---|---|---|---|
| Phosphorus, mg/dL | 5.4 ± 0.9a | 6.5 ± 1.2a,b | 7.4 ± 0.9b | 6.4 ± 0.7a,b | 6.4 ± 0.9a,b | 0.002 |
| Glucose, mg/dL | 148.3 ± 91.7a | 205.0 ± 57.5a,b | 245.4 ± 83.3a,b | 263.9 ± 68.6b | 277.0 ± 76.5b | 0.004 |
| Insulin, ng/mL | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.8 ± 0.8 | 0.8 ± 0.9 | 0.7 ± 0.9 | 0.487 |
| TG, mg/dL | 35.7 ± 6.7 | 41.2 ± 17.2 | 45.0 ± 22.7 | 40.6 ± 15.8 | 41.2 ± 13.1 | 0.807 |
| Cholesterol, mg/dL | 87.0 ± 22.1 | 85.3 ± 13.3 | 84.8 ± 14.7 | 85.5 ± 15.9 | 105.4 ± 25.3 | 0.081 |
| Albumin, mg/dL | 3.2 ± 0.2 | 3.2 ± 0.3 | 3.1 ± 0.2 | 3.1 ± 0.2 | 3.2 ± 0.3 | 0.921 |
| C-reactive protein, mg/dL | 3.2 ± 0.4 | 2.8 ± 0.4 | 2.7 ± 0.7 | 2.7 ± 0.8 | 3.1 ± 0.3 | 0.162 |
Values are means ± SDs. Significance was set at P < 0.05. Categories in the same row that do not share the same superscript letters are significantly different. LP-0.015P, 10% protein and 0.015% P; LP-0.056P, 10% protein and 0.056% P; LP-0.1P, 10% protein and 0.1% P; LP-0.3P, 10% protein and 0.3% P; NP-0.3P, 20% protein and 0.3% P (control group).
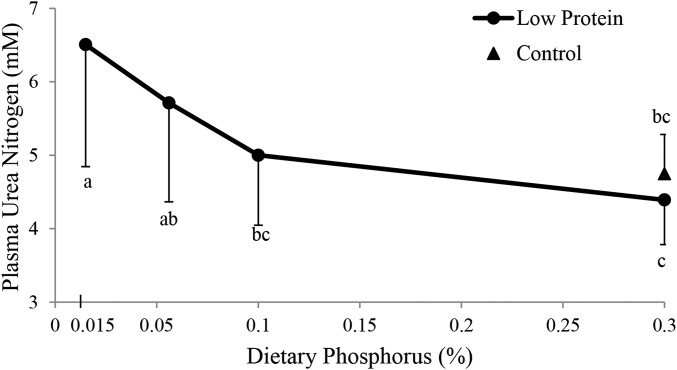
In the low-protein treatment groups, plasma urea nitrogen decreased when the phosphorus content of the diet increased to ≤0.1% P. No differences in plasma urea nitrogen were observed among the NP-0.3P, LP-0.1P, and LP-0.3P groups (Figure 2).
FIGURE 2.
Plasma urea nitrogen concentrations (expressed in mM) of rats fed a control diet or 1 of 4 low-protein diets with different phosphorus concentrations for 9 wk. Triangles indicate the control group (20% protein and 0.3% P). Circles indicate the low-protein groups (10% protein with 0.015% P, 0.056% P, 0.1% P, or 0.3% P). The plasma urea nitrogen concentrations are expressed as means ± SDs as a function of the level of phosphorus in the diet (n = 9 or 10). One-factor ANOVA and specific comparisons were made between each of the 5 groups using Fisher's pairwise comparisons. Values that do not share the same superscript letter are significantly different (P < 0.05).
Liver histology
Analyses of liver photomicrographs (Supplemental Figure 2) indicated that steatosis grade and location, microvesicular steatosis, and portal inflammation were similar among groups and thus were not affected by the protein or phosphorus content of the diet. All groups had approximately similar cases of microvesicular steatosis. Approximately 5–6 rats from each group presented with grade 0 or grade 1 steatosis, and only 1 rat from each group developed grade 3 steatosis. No signs of Mallory hyaline bodies, fibrosis, or glycogenated nuclei were detected in all rats. Only lobular inflammation was significantly greater in the low-protein groups than in the normal dietary protein group (Table 3).
TABLE 3.
Liver histology results of rats fed a control diet or 1 of 4 low-protein diets with different phosphorus concentrations for 9 wk1
| Variable | LP-0.015P (n = 9) | LP-0.056P (n = 10) | LP-0.1P (n = 10) | LP-0.3P (n = 10) | NP-0.3P (n = 10) | ANOVA P value |
|---|---|---|---|---|---|---|
| Oil Red O image analysis, µm2 | 280 ± 727 | 1510 ± 2837 | 1234 ± 1636 | 2075 ± 3088 | 664 ± 1457 | 0.407 |
| Steatosis grade | 0.4 ± 1.0 | 0.5 ± 1.0 | 0.9 ± 1.1 | 0.6 ± 1.0 | 0.3 ± 0.7 | 0.701 |
| Location | 0.7 ± 1.0 | 0.7 ± 1.3 | 1.8 ± 1.6 | 0.9 ± 1.5 | 0.3 ± 0.9 | 0.118 |
| Microvesicular steatosis | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.9 ± 0.3 | 0.7 ± 0.5 | 0.5 ± 0.5 | 0.385 |
| Lobular inflammation | 0.7 ± 0.9a,b | 0.7 ± 1.0a,b | 1.4 ± 1.1a | 0.5 ± 0.7b | 0.1 ± 0.3b | 0.020 |
| Portal inflammation | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.895 |
Values are means ± SDs. One-factor ANOVA was used to detect significant differences between groups. Significance was set at P < 0.05. Categories in the same row that do not share the same subscripts are significantly different. LP-0.015P, 10% protein and 0.015% P; LP-0.056P, 10% protein and 0.056% P; LP-0.1P, 10% protein and 0.1% P; LP-0.3P, 10% protein and 0.3% P; NP-0.3P, 20% protein and 0.3% P (control group).
Discussion
This study was designed to investigate the impact of dietary phosphorus supplementation on several growth parameters of rats maintained with a low-protein diet. In animals, food intake was regulated to serve 2 objectives. First, satisfying nutritional needs for growth and maintenance entails long-term regulation of intake, which is usually accompanied by an increase in food intake. In support of this, diet selection and protein restriction studies show that young animals are capable of adjusting their intake to support their nutritional protein requirements (29, 30) for growth and maintenance. Second, maintenance of homeostasis relates to acute or short-term regulation of food intake (31), which is usually associated with a decrease in food intake to avoid the toxicity of amino acid accumulation that is common with the ingestion of low-quality proteins. Thus, reduced food intake of the low-protein low-phosphorus diet groups (LP-0.015P and LP-0.056P) compared with the other low-protein groups (LP-0.1P and LP-0.3P), despite the similarity in dietary protein contents, is likely to have resulted from a dietary adaptation for the maintenance of homeostasis (32–34). Adequate protein metabolism in terms of synthesis and degradation depends on the availability of essential amino acids and energy needed to catalyze these reactions. Protein synthesis is a high energy-requiring process, in which 4 ATP equivalents are required for the formation of 1 peptide bond, or the equivalent of 0.67 kcal/1 g of protein synthesized (30). Several observations indicate that this process is highly dependent on phosphorus availability (35–37). In mice, dietary phosphorus restriction was reported to lower gastrocnemius muscle tension owing to a slow rate of ATP synthesis, and this was reversed by phosphorus supplementation (35). In addition, ATP depletion by 2.4-dinitrophenol (36) or fructose infusion (37) was reported to reduce protein synthesis. Hence, under the condition of low phosphorus intake, the body loses its capacity to synthesize protein as a result of reduced ATP availability that is dependent on exogenous phosphorus replenishment (35, 36) and amino acids ultimately accumulate in the circulation. Consequently, food intake is reduced to protect against amino acid toxicity. The ability of phosphorus to alter protein metabolism is supported by the fact that dietary phosphorus content is inversely related to plasma urea nitrogen. Thus, it can be concluded that the observed increase in food intake with the addition of phosphorus to a low-protein diet is likely attributable to an improvement in amino acid homoeostasis owing to enhanced protein metabolism (e.g., protein synthesis). The fact that proteins are the main sources of dietary phosphorus and that bodily protein metabolism is dependent on phosphorus makes it reasonable to postulate that reduced food intake under low-protein diets (38) is highly related to the alteration of amino acid homeostasis in a manner that mimics that of a low-quality protein diet. Accordingly, the reported changes in food intake after protein (casein) manipulation (10) may have been partially related to the phosphorus content of the diet, especially because 50% of phosphorus (0.15% of diet) in the rodents' diet is derived from casein (24).
On the other hand, measures of body composition seem to be highly dependent on phosphorus availability in the diet, as indicated by the resemblances in body composition between the LP-0.3P and NP-0.3P groups. This is further supported by findings in which differences in body weight between the normal phosphorus-containing (LP-0.3P and NP-0.3P) and the phosphorus-depleted (LP-0.015P and LP-0.056P) groups were associated with changes in both body fat and protein. For example, the 10% reduction in the percentage of body water in the NP-0.3P and LP-0.3P groups was almost replaced by a 5% increase in the percentage of body fat and a 5% increase in the percentage of protein, whereas the opposite was true for the phosphorus-depleted groups (LP-0.015P and LP-0.056P). In the latter, the sharp reduction in weight gain was associated with an increase in the percentage of body water. Increased water retention is known to be the main feature of kwashiorkor, which was not explained by protein restriction alone (10, 39) and is known to be associated with hypophosphatemia (40) and poor dietary phosphorus availability (41). The inability of phosphorus restriction to increase hepatic water retention argues against its involvement in the development of kwashiorkor. It is worth noting that body composition changes after phosphorus manipulation were mainly attributed to energy efficiency, because the magnitude of changes in food intake was modest.
In addition, the high resemblance between the LP-0.1P and control (LP-0.3P and NP-0.3P) groups in terms of weight gain, food intake, and body weight was not reflected by similarities in body composition, because the percentage of body protein (defatted percentage) of the 0.3% P (LP-0.3P and NP-0.3P) groups was higher than that of the LP-0.1P group. This further confirms the importance of phosphorus in protein metabolism and indicates that phosphorus is capable of altering body composition. In this study, no significant alterations in liver composition and histology were detected, despite the fact that dietary phosphorus restriction in mice is reported to increase hepatic lipid accumulation, especially under conditions of increased cholesterol content in the diet (42).
Moreover, the failure of dietary phosphorus restriction to significantly affect plasma phosphorus content except under extreme conditions of restriction (LP-0.015P) is in line with findings in humans (43), in which plasma phosphorus is reported to not be an indicator of phosphorus intake. However, manipulation of dietary phosphorus (between 0.056% and 0.3% P) affected food intake, energy efficiency, weight gain, and body composition, although plasma phosphorus was not affected. This further confirms that the plasma phosphorus concentration is neither a good marker of phosphorus intake nor an indicator of changes in food intake or weight measures.
Furthermore, the elevated plasma glucose observed in the control group may have been related to the effect of egg white protein on insulin secretion (44–46). Human studies have shown that the incremental insulin area was much lower in both healthy subjects (45) and individuals with diabetes (44) after ingestion of 50 g of egg white compared with cottage cheese. In addition, ingestion of a breakfast with whole egg, egg white, or egg yolk was associated with an increase in blood glucose concentration after egg white ingestion than after meals with whole egg or egg yolk (46). However, the resemblance in plasma glucose between LP-0.3P and NP-0.3P implies that plasma glucose is not solely dependent on the quantity of egg white in the diet. In low-protein (egg white) groups, the increase in plasma glucose with the addition of phosphorus possibly indicates that egg white ingestion is able to blunt the effect of phosphorus on insulin sensitivity (47) or the presence of varied glycogen storage between the groups. The latter option may prevail, because plasma glucose is affected by hepatic glycogen content that is known to be stimulated by phosphorus intake (48).
The ability of phosphorus intake to manipulate food intake and body composition requires further investigations to determine the level of phosphorus intake that is capable of improving body composition.
Low body protein content (defatted percentage) was present under conditions of 0.1% P (LP-0.1P; ∼0.25 mg P/kcal) and was lower despite normal protein intake, whereas higher levels of phosphorus intake improved body protein content. The level of phosphorus intake (0.25 mg/kcal of highly bioavailable phosphorus) resembles that consumed by most people, which is ∼0.5 mg P/kcal (49) assuming a 50% bioavailability (owing to the significant contribution of plant sources). This level is not thought to enhance protein status. In support of this, a recent study in humans reported an improvement in body weight and waist circumference of overweight and obese subjects after 12 wk of phosphorus supplementation (50). Although body composition was not measured, an increase in the percentage of lean body mass would be expected.
Under moderate protein restriction (10%), the addition of phosphorus (0.3%) was able to significantly improve food intake, weight gain, and energy efficiency similar to a normal protein diet (20%) containing the same concentration of phosphorus (0.3%). Body protein content (defatted percentage) was high with a 0.3% P diet, irrespective of protein content (10% or 20%). The pattern of changes in the different measures seems to indicate that a dietary phosphorus content of 0.1% may represent a critical threshold. However, it is not clear whether phosphorus would be able to exert these effects under added protein restriction. Our results may have implications on the management of malnutrition and may have financial value, because the cost of protein is much higher than that of phosphorus. In addition, our findings suggest that not only the protein quantity but also the phosphorus content of the diet is of extreme importance for improving food intake, weight gain, and energy efficiency.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—OAO: designed the study, performed the statistical analysis, supervised the work, and had primary responsibility for the final content; RUH: collected the data and wrote the draft manuscript; OAO and RUH: analyzed the data; MNJ, ANT, and HG: critically revised the manuscript for important intellectual content; and all authors: read and approved the final manuscript.
Abbreviations
- H&E
hematoxylin and eosin
- LP-0.015P
10% protein and 0.015% P
- LP-0.056P
10% protein and 0.056% P
- LP-0.1P
10% protein and 0.1% P
- LP-0.3P
10% protein and 0.3% P
- NP-0.3P
20% protein and 0.3% P
- ORO
Oil Red O
Footnotes
Supported by a grant from the University Research Board at the American University of Beirut.
References
- 1. Wu G.. Dietary protein intake and human health. Food Funct 2016;7:1251–65. [DOI] [PubMed] [Google Scholar]
- 2. Geraghty AA, Lindsay KL, Alberdi G, McAuliffe FM, Gibney ER.. Nutrition during pregnancy impacts offspring's epigenetic status—evidence from human and animal studies. Nutr Metab Insights 2016;8(Suppl 1):41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langley-Evans S, Sherman R, Welham S, Jackson A... Fetal exposure to maternal low protein diets during discrete periods of pregnancy induces hypertension in the rat. Clin Sci 1997;92:13P. [DOI] [PubMed] [Google Scholar]
- 4. Bhasin KKS, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ.. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 2009;58:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyer JH.. Interactions of dietary fiber and protein on food intake and body composition of growing rats. Am J Physiol 1958;193:488–94. [DOI] [PubMed] [Google Scholar]
- 6. Noblet J, Henry Y, Dubois S.. Effect of protein and lysine levels in the diet on body gain composition and energy utilization in growing pigs. J Anim Sci 1987;65:717–26. [DOI] [PubMed] [Google Scholar]
- 7. White BD, He B, Dean RG, Martin RJ.. Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J Nutr 1994;124:1152–60. [DOI] [PubMed] [Google Scholar]
- 8. White BD, Dean RG, Martin RJ.. An association between low levels of dietary protein, elevated NPY gene expression in the basomedial hypothalamus and increased food intake. Nutr Neurosci 1998;1:173–82. [DOI] [PubMed] [Google Scholar]
- 9. Meyer J, Hargus W.. Factors influencing food intake of rats fed low-protein rations. Am J Physiol 1959;197:1350–2. [Google Scholar]
- 10. Du F, Higginbotham DA, White BD.. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr 2000;130:514–21. [DOI] [PubMed] [Google Scholar]
- 11. Harper AE.. Effect of variations in protein intake on enzymes of amino acid metabolism. Can J Biochem 1965;43:1589–603. [DOI] [PubMed] [Google Scholar]
- 12. Muramastu K, Ashida K.. Relationship between the nutritive value of dietary protein and liver xanthine oxidase activity in young rats. Agric Biol Chem 1962;26:25–9. [Google Scholar]
- 13. Colombo J-P, Cervantes H, Kokorovic M, Pfister U, Perritaz R.. Effect of different protein diets on the distribution of amino acids in plasma, liver and brain in the rat. Ann Nutr Metab 1992;36:23–33. [DOI] [PubMed] [Google Scholar]
- 14. Beck B, Dollet J-M, Max J-P.. Refeeding after various times of ingestion of a low protein diet: effects on food intake and body weight in rats. Physiol Behav 1989;45:761–5. [DOI] [PubMed] [Google Scholar]
- 15. Boaz M, Smetana S.. Regression equation predicts dietary phosphorus intake from estimate of dietary protein intake. J Am Diet Assoc 1996;96:1268–70. [DOI] [PubMed] [Google Scholar]
- 16. Amanzadeh J, Reilly RF.. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol 2006;2:136–48. [DOI] [PubMed] [Google Scholar]
- 17. Solomon SM, Kirby DF.. The refeeding syndrome: a review. JPEN J Parenter Enteral Nutr 1990;14:90–7. [DOI] [PubMed] [Google Scholar]
- 18. Morris RC Jr., Nigon K, Reed EB.. Evidence that the severity of depletion of inorganic phosphate determines the severity of the disturbance of adenine nucleotide metabolism in the liver and renal cortex of the fructose-loaded rat. J Clin Invest 1978;61:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waterlow JC.. Protein-energy malnutrition: the nature and extent of the problem. Clin Nutr 1997;16(Suppl 1):3–9. [DOI] [PubMed] [Google Scholar]
- 20. Ravindran V, Ravindran G, Sivalogan S.. Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem 1994;50:133–6. [Google Scholar]
- 21. Pozrl T, Kopjar M, Kurent I, Hribar J, Janes A, Marian S.. Phytate degradation during breadmaking: the influence of flour type and breadmaking procedures. Czech J Food Sci 2009;27:29–38. [Google Scholar]
- 22. Lopez HW, Krespine V, Guy C, Messager A, Demigne C, Remesy C.. Prolonged fermentation of whole wheat sourdough reduces phytate level and increases soluble magnesium. J Agric Food Chem 2001;49:2657–62. [DOI] [PubMed] [Google Scholar]
- 23. US Environmental Protection Agency EPA 200-7/8. Methods for the determination of metals in environmental samples. Washington (DC): US Environmental Protection Agency; 1991. [Google Scholar]
- 24. Murai I, Shukuin S, Sugimoto M, Ikeda S, Kume S.. Effects of high potassium chloride supplementation on water intake and bodyweight gains in pregnant and lactating mice. Anim Sci J 2013;84:502–7. [DOI] [PubMed] [Google Scholar]
- 25. Jodas EM, Voltera AF, Ginoza M, Kohlmann O Jr., dos Santos NB, Cesaretti ML.. Effects of physical training and potassium supplementation on blood pressure, glucose metabolism and albuminuria of spontaneously hypertensive rats. J Bras Nefrol 2014;36:271–9. [PubMed] [Google Scholar]
- 26. Reeves PG, Nielsen FH, Fahey GC Jr... AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 27. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 28. Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A.. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 2013;8:1149–54. [DOI] [PubMed] [Google Scholar]
- 29. Leathwood PD, Ashley DV.. Strategies of protein selection by weanling and adult rats. Appetite 1983;4:97–112. [DOI] [PubMed] [Google Scholar]
- 30. Shariatmadari F, Forbes J.. Growth and food intake responses to diets of different protein contents and a choice between diets containing two concentrations of protein in broiler and layer strains of chicken. Br Poult Sci 1993;34:959–70. [DOI] [PubMed] [Google Scholar]
- 31. Radcliffe JD, Webster AJ.. Regulation of food intake during growth in fatty and lean female Zucker rats given diets of different protein content. Br J Nutr 1976;36:457–69. [DOI] [PubMed] [Google Scholar]
- 32. Kabadi UM, Eisenstein AB, Strack I.. Decreased plasma insulin but normal glucagon in rats fed low protein diets. J Nutr 1976;106:1247–53. [DOI] [PubMed] [Google Scholar]
- 33. Peters JC, Harper AE.. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr 1985;115:382–98. [DOI] [PubMed] [Google Scholar]
- 34. Heard CR, Frangi SM, Wright PM, McCartney PR.. Biochemical characteristics of different forms of protein-energy malnutrition: an experimental model using young rats. Br J Nutr 1977;37:1–21. [DOI] [PubMed] [Google Scholar]
- 35. Hettleman BD, Sabina RL, Drezner MK, Holmes EW, Swain JL.. Defective adenosine triphosphate synthesis. An explanation for skeletal muscle dysfunction in phosphate-deficient mice. J Clin Invest 1983;72:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fischbeck KH.. Effects of ATP depletion and protein synthesis inhibition on muscle plasma membrane orthogonal arrays. Exp Neurol 1984;83:577–88. [DOI] [PubMed] [Google Scholar]
- 37. Bode JC, Zelder O, Rumpelt H, Wittkampy U.. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest 1973;3:436–41. [DOI] [PubMed] [Google Scholar]
- 38. Peng Y, Gubin J, Harper A, Vavich M, Kemmerer A.. Food intake regulation: amino acid toxicity and changes in rat brain and plasma amino acids. J Nutr 1973;103:608–17. [DOI] [PubMed] [Google Scholar]
- 39. Golden MH.. Protein deficiency, energy deficiency, and the oedema of malnutrition. Lancet 1982;319:1261–5. [DOI] [PubMed] [Google Scholar]
- 40. Kimutai D, Maleche-Obimbo E, Kamenwa R, Murila F.. Hypo-phosphataemia in children under five years with kwashiorkor and marasmic kwashiorkor. East Afr Med J 2009;86:330–6. [DOI] [PubMed] [Google Scholar]
- 41. Manary MJ, Heikens GT, Golden M.. Viewpoint: part 3: kwashiorkor: more hypothesis testing is needed to understand the aetiology of oedema. Malawi Med J 2009;21:106–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka S, Yamamoto H, Nakahashi O, Kagawa T, Ishiguro M, Masuda M, Kozai M, Ikeda S, Taketani Y, Takeda E.. Dietary phosphate restriction induces hepatic lipid accumulation through dysregulation of cholesterol metabolism in mice. Nutr Res 2013;33:586–93. [DOI] [PubMed] [Google Scholar]
- 43. de Boer IH, Rue TC, Kestenbaum B.. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009;53:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gannon MC, Nuttall FQ, Lane JT, Burmeister LA.. Metabolic response to cottage cheese or egg white protein, with or without glucose, in type II diabetic subjects. Metabolism 1992;41:1137–45. [DOI] [PubMed] [Google Scholar]
- 45. Nuttall FQ, Gannon MC.. Metabolic response to egg white and cottage cheese protein in normal subjects. Metabolism 1990;39:749–55. [DOI] [PubMed] [Google Scholar]
- 46. Pelletier X, Thouvenot P, Belbraouet S, Chayvialle J, Hanesse B, Mayeux D, Debry G.. Effect of egg consumption in healthy volunteers: influence of yolk, white or whole-egg on gastric emptying and on glycemic and hormonal responses. Ann Nutr Metab 1996;40:109–15. [DOI] [PubMed] [Google Scholar]
- 47. Khattab M, Abi-Rashed C, Ghattas H, Hlais S, Obeid O.. Phosphorus ingestion improves oral glucose tolerance of healthy male subjects: a crossover experiment. Nutr J 2015;14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie W, Tran TL, Finegood DT, van de Werve G.. Dietary P(i) deprivation in rats affects liver cAMP, glycogen, key steps of gluconeogenesis and glucose production. Biochem J 2000;352:227–32. [PMC free article] [PubMed] [Google Scholar]
- 49. Chang AR, Lazo M, Appel LJ, Gutiérrez OM, Grams ME.. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr 2014;99:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ayoub JJ, Samra MJ, Hlais SA, Bassil MS, Obeid OA.. Effect of phosphorus supplementation on weight gain and waist circumference of overweight/obese adults: a randomized clinical trial. Nutr Diabetes 2015;5:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.