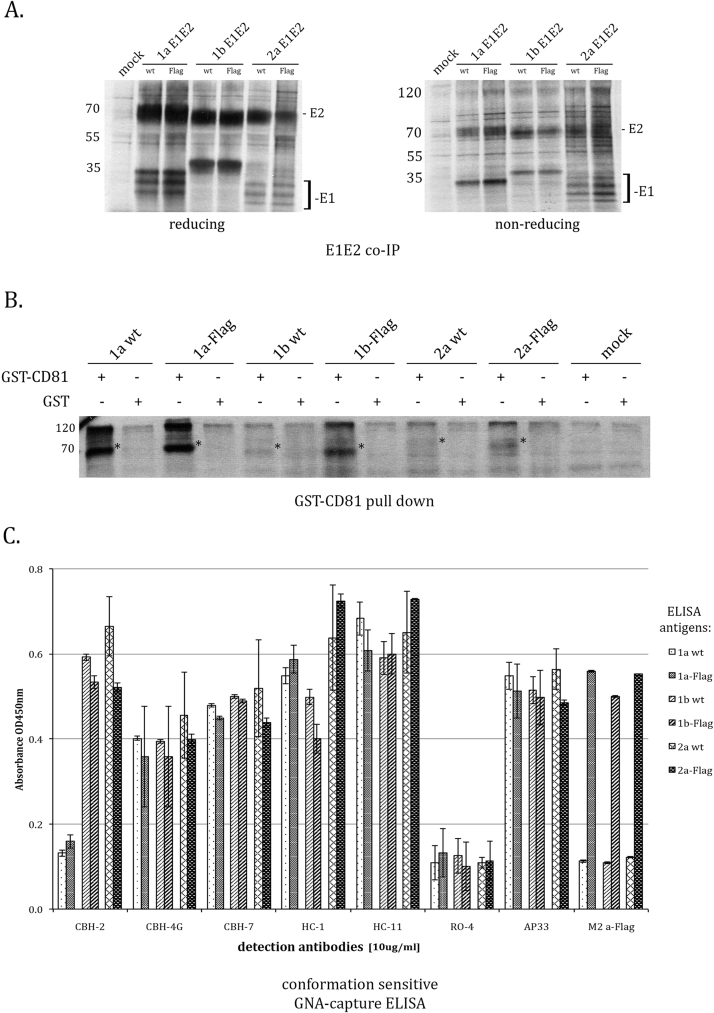
Fig. 3.
Functional and structural analyses of E1E2-Flag proteins. A. E1 glycoprotein co-immunoprecipitates with E2. 293T cells transiently expressing wt and E1E2-Flag were metabolically radiolabeled with [35S]-Met and lysed 48 hrs post transfection. The proteins were immunoprecipitated with the anti-E2 AP33 mAb, separated on 12% SDS-PAGE (under reducing and non-reducing conditions) and detected by radiography. B. Binding of E1E2-Flag to CD81 tested by pull-down assay. GST-hCD81-LEL was bound to glutathione agarose, the batch was divided into aliquot parts and incubated with lysates of 293T cell expressing HCV E1E2. GST bound to glutathione agarose was used as a negative control. The samples were resolved on 12% SDS-PAGE gel and detected with AP33 mAb. Asterisks indicate the position of E2. C. Analysis of conformational epitopes of HCV E1E2-Flag. Lectin-bound E1E2 complexes from transfected 293T cell lysates were probed with a panel of conformation-sensitive anti-E2 antibodies. An irrelevant isotype control hmAb RO4 and the slightly conformation-sensitive anti-E2 mAb AP33 were used as negative and positive controls, respectively. Bars represent the mean values obtained from triplicate experiments; standard deviation of the readings is indicated.