Abstract
With rapid biotechnological advances in specialty drugs and direct-to-consumer advertising, consumers are under tremendous pressure to look, perform, feel, and live better. This is often accomplished through the use of life-enhancing products, sometimes referred to as performance-enhancing products, which can be accessed only through a gatekeeper, such as a physician. Integrating consumer and medical research, this article investigates how physicians make trade-offs between objective medical and nonmedical factors to determine consumers’ access to life-enhancing products by examining US pediatric endocrinologists’ prescription decisions for growth hormone (GH) for healthy but short children. The results of a conjoint study indicate that consumer medical criteria have less impact on a physician’s decision to prescribe GH if the consumer requests a prescription or the physician believes in the intangible product benefits, and more impact when the product is more expensive. A physician’s length of experience increases the impact of consumer medical criteria and decreases the influence of a consumer’s preference for a prescription on the decision to prescribe. Overall, this research shows that not all consumers have equal access to life-enhancing products; their access depends on a complex combination of medical and nonmedical factors related to the consumer, product, and the physician.
Keywords: consumer access, life-enhancing, performance-enhancing, decision making, prescription, growth hormone, public policy
INTRODUCTION
In the fourth grade, Angelo Tufano was so short he looked like a kindergartner, and he was miserable.“I was picked on a lot. Other kids called me ‘midget’ and ‘shrimp,’”says Angelo, now an 18-year-old student at Columbia College in Chicago.“I used to cry.”His mother, Carmela, persuaded an endocrinologist to prescribe a growth hormone, even though tests showed he didn’t qualify for a prescription. Eventually she talked her insurance company into dropping the monthly co-pay for the drug from $700 a month to $5. Now Angelo stands at 5-foot-9—a full 10 inches taller than his doctors predicted he’d be without the drug.“I was worried society wouldn’t accept him,”Carmela says.“As a parent, you do what you have to do.”
—BusinessWeek (Weintraub and Arndt 2005)
Josh and Matt are 10 years old and are healthy but short and growing slowly for their age (–2 SD on the standard growth chart). Their families discover that daily injections of growth hormone (GH)—though very expensive and with unknown side effects—may add extra inches of height. To receive GH, they need a prescription from a pediatric endocrinologist (for simplicity, we refer to them as physicians hereafter). Each family visits their respective physicians. Although Josh and Matt have the same medical criteria, Josh’s parents walk out of the physician’s office with a GH prescription in hand, whereas Matt’s physician decides not to prescribe GH. Why does one child get a chance to grow taller while the other does not? This study examines factors that lead to such different outcomes. Specifically, we consider how physicians make trade-offs between medical and nonmedical factors in deciding which consumers get access to life-enhancing products (e.g., GH for healthy but short children).
A life-enhancing product is one aimed not to cure a disease but to augment or improve natural capacities (i.e., “normal” workings) of the human body and mind (Allen and Fost 2004). Life-enhancing products, sometimes referred to as performance-enhancing, have become extremely popular due to rapid biotechnological advances and direct-to-consumer advertising of developed products, which impose significant pressure on consumers to look, perform, feel, and live better (President’s Council on Bioethics 2003). The market for life-enhancing products is enormous and growing, involving tens of billions of dollars in sales (see table 1). Millions of consumers turn to injections and plastic surgery to improve their appearance (American Society of Plastic Surgeons 2013), stimulants to enhance cognitive performance (Riis, Simmons, and Goodwin 2008), or growth hormone to increase their height (Weintraub and Arndt 2005), without any objective medical need. In the United States consumers spend about 25 times more (approximately $2.4 billion) on injections of Botox or Dysport to minimize the appearance of wrinkles than is spent on research to cure malaria, a disease affecting hundreds of millions of people (American Society of Plastic Surgeons 2013; President’s Council on Bioethics 2003). Spending on elective plastic surgery in the United States has never been higher, reaching $12.6 billion in 2013 (American Society of Plastic Surgeons 2013). In their recent study, Williams and Steffel (2014) investigated consumer preferences for life-enhancing product use from a consumer and public policy perspective, highlighting the vast scale and scope of life-enhancing products. However, despite the growth and importance of this unique market, the consumption of life-enhancing products is not well understood by marketing or medical researchers.
TABLE 1.
CHARACTERISTICS AND MARKET SIZE OF LIFE-ENHANCING PRODUCTS
| Life-Enhancing Purpose | Traditional Therapy Purpose | |
|---|---|---|
| Substantive Elements | ||
| Related to consumer and product | ||
| Consumer health status | Healthy (Juengst 1997) | Sick (Juengst 1997) |
| Objective medical necessity for product | Unnecessary (Allen and Fost 2004; Williams and Steffel 2014) | Necessary (Allen and Fost 2004; Williams and Steffel 2014) |
| Consumer objective | Improve oneself beyond the full potential of one's body and mind (Williams and Steffel 2014) | Perform back up to one's full potential when suffering from an illness or disability (Williams and Steffel 2014) |
| Consumer involvement | Higher (Camacho et al. 2014) | Lower (Camacho et al. 2014) |
| Related to decision-making | ||
| Decision timeframe | Longer/more flexible/less defined (Heden et al. 2009) | Shorter/ less flexible/more defined (Heden et al. 2009) |
| Physician's functions | More of a gatekeeper (Colsman et al. 2005; Lee 2006) | More diagnostician/advisor (Colsman et al. 2005; Lee 2006) |
| Medical guidelines for treatment | Less clear (Allen and Fost 2004; Lee 2006) | More clear (Allen and Fost 2004; Lee 2006) |
| Market Size and Use | ||
| Amphetamine-type stimulants (Adderall, Ritalin, etc.) | Enhancement of cognitive performance; 33 million users worldwide in 2012 (World Drug Report 2012) | Attention deficit disorder; attention deficit hyperactivity disorder; market size unavailable |
| Breast implants | Breast augmentation; $1.1 billion; 290,000 procedures in the U.S. in 2013; (Plastic Surgery Statistics Report 2013) | Breast reconstruction after breast cancer; 96,000 procedures in the U.S. in 2013 (Plastic Surgery Statistics Report 2013) |
| Growth hormone | Increasing height of short but healthy children; potentially 500,000 U.S. children in 2010 (Silvers et al. 2010) | GH deficiency, Turner and short bowel syndromes, kidney insufficiency, muscle-wasting disease; potentially 24,000 U.S. children with the top three conditions (Finkelstein et al. 1998) |
Life enhancement practices are driven by consumer-dictated (not medical) needs and wants, yet access to these products is determined by a medical gatekeeper (physician). In deciding whether to prescribe a particular life-enhancing product, a physician takes into consideration two types of factors: medical, such as the patient’s labs, medical history, and the feasibility of the treatment for that particular patient, and nonmedical factors, such as consumer preferences, intangible product benefits, and product expensiveness (Hajjaj et al. 2010). Thus, physicians often face difficult trade-offs in that they must ensure medical feasibility while simultaneously addressing consumers’ life-enhancing needs, such as reducing wrinkles or increasing height. To the best of our knowledge, no research has examined how physicians resolve these trade-offs involving medical and nonmedical factors when making decisions about whether to provide access to life-enhancing products. Despite this lack of research, medical academics conclude that nonmedical factors are the “biggest obstacle” to medical decision making, because they are poorly understood (Hajjaj et al. 2010). Little is known about how nonmedical factors influence medical decision making in general and for life-enhancing products in particular—a context in which nonmedical factors likely assume great importance.
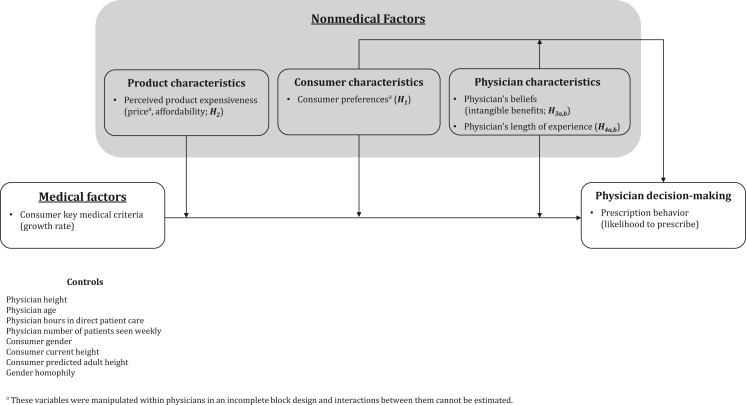
In this research, we investigate how physicians make trade-offs among medical and nonmedical factors, related to the consumer, product, and the physician, when they decide whether to prescribe GH, the first biotechnological drug approved by the US Food and Drug Administration (FDA) for life-enhancing purposes. Specifically, our conceptual framework shows how physicians make trade-offs between consumers’ medical criteria, consumers’ preferences for a prescription, product expensiveness, and physician-related factors when deciding whether to prescribe a life-enhancing product. We empirically test this framework with a conjoint study involving a census of US physicians who prescribe GH for healthy but short children. The results show that consumers’ preference for a life-enhancing prescription and physicians’ belief in the intangible product benefits weaken the impact of consumers’ objective medical criteria on the likelihood of product prescription, whereas high product expensiveness strengthens it. Physicians’ length of experience plays a moderating role by increasing the impact of consumer medical criteria and decreasing the influence of consumers’ preference for prescription on the likelihood that a physician will prescribe the product. Overall, this research shows that not all consumers receive equal access to life-enhancing products; instead, access depends on medical and nonmedical factors related to the consumer, product, and physician as well as on the interactions between them.
RESEARCH BACKGROUND: LIFE-ENHANCING PRODUCTS AND PHYSICIAN DECISION MAKING
Life-enhancing products differ from most consumer products in that access to them depends on a gatekeeper. They also differ from medical products in accordance with the “objective distinction between curative treatments and enhancement uses of ordinary medical services like psychotherapy and prescriptions of human growth hormone” (Juengst 1997, 129). Table 1 summarizes these differences. Consumers seeking a product for life-enhancing purposes are healthy, with no objective medical need for a product or procedure (Allen and Fost 2004; Juengst 1997). Consumer research clarifies this distinction as follows: when considering a product for a life-enhancing purpose, the consumer’s goal is to improve the self beyond its full potential, whereas a consumer suffering from a medical disease wants to get back up to his or her full potential (Williams and Steffel 2014). Although many life-enhancing products were first developed to address medical diseases, their predominant uses have shifted to life-enhancing purposes. For example, GH “can be compared with other technologies including artificial insemination and Prozac, which began as treatments for serious medical problems but expanded to much larger markets that involved problems that were less clearly medical” (Allen and Fost 2004, 651). In many cases, as table 1 illustrates, the life-enhancing market is much larger than the traditional therapy market for a given product.
As with traditional medical therapies consumers typically need a physician to gain access to life-enhancing products. However, relative to traditional therapies, consumers are usually more engaged in the decision process when it comes to life-enhancing products, which often reduces the role of the physician to that of a gatekeeper. The World Health Organization (2008) reports that healthier people, such as consumers seeking life-enhancing products, want to be more involved in the decision-making process than those who are very ill, across various health conditions. Botti, Orfali, and Iyengar (2009) find that people facing highly undesirable, consequential, and unavoidable medical decisions experience reduced coping abilities and are not very involved in decision making. Hedén et al. (2009) show that the overwhelming majority of women considering breast implants for a life-enhancing purpose (80%) want to be thoroughly involved in all aspects of the decision-making process and not leave decisions to the physician. In contrast, only 22% of women with breast cancer seek such involvement (Hitz et al. 2013). Medical literature similarly concludes that the role of the physician in the case of life-enhancing products gets largely reduced to a gatekeeping function, rather than diagnostician or therapist roles (Colsman et al. 2005).
Further, the consumer’s timeframe for making a decision about using a life-enhancing product tends to be longer and more flexible than for traditional medical therapies. Therefore, consumers have relatively more opportunity to collect extensive information about life-enhancing products and spend substantial time forming and validating their product preferences (Hedén et al. 2009; Hitz et al. 2013). For example, women research and deliberate an average of four years before undergoing breast augmentation surgery—one of the most popular elective surgeries in the United States (American Society of Plastic Surgeons 2013; Hedén et al. 2009).
This summary suggests two prominent knowledge gaps in the consumer and medical research literatures. First, despite widespread agreement that “an ideal patient–physician relationship should be characterized by good interaction between two experts: the physician, who is an expert on diagnoses and treatments, and the patient, who is the expert on his or her values and preferences and how the disease interferes with his or her life” (Camacho, Landsman, and Stremersch 2010, 121), it is unclear how physicians incorporate consumer-related factors, such as the consumer’s preference for prescription, into their treatment decisions. Second, though physician-related factors, such as intuition, beliefs, and length of experience, likely influence treatment decisions, the role of these factors has not been studied systematically. Medical literature indicates that the varying influence of consumer, product, and physician factors means that “patients are still unlikely to receive equality of treatment” (Hajjaj et al. 2010, 185). This inequality may be even more prevalent for life-enhancing treatments where medical guidelines are less clear, leaving space for a wider range of potential applications of these guidelines due to the influences of the preceding categories of factors (Allen and Fost 2004; Lee 2006).
HYPOTHESES
Medical literature identifies two broad categories of factors that drive physician decision making in general: medical and nonmedical (Hajjaj et al. 2010). Medical factors are criteria based on evidence of effectiveness across consumers and might include a consumer’s medical history or the results of various diagnostic tests and labs (Eddy 2005). For example, in the context of this research, the key medical criterion for prescribing GH to healthy but short children is the child’s growth rate (Radovick and McGillivray 2013, Silvers et al. 2010). Most children who are evaluated for a GH prescription are growing slowly, though there is a variation in growth rate among them typically ranging from slow to very slow. A growth rate that corresponds to deviating from the growth curve (e.g., –3 SD) is considered very slow, an indication that GH is clinically appropriate and feasible. A slow but faster growth rate (e.g., –1 SD) that parallels the growth curve reduces the likelihood of GH prescription. In other words, children who are better off in terms of their medical criteria (i.e., growing faster) are less likely to get a GH prescription. This constitutes a negative main effect of consumer medical criteria on the likelihood of GH prescription, which is well established in the medical literature (Silvers et al. 2010) and is not included in our formal hypotheses. Thus, the moderating hypotheses in this research build upon this negative main effect, which indicates that faster growth rates will reduce the likelihood of a GH prescription.
As recently as 2010, researchers noted that the influence of nonmedical factors on physicians’ decision making is important but poorly understood, even in traditional medicine research (Hajjaj et al. 2010). Nonmedical factors include consumer-related characteristics, such as consumer preference for prescription; product characteristics, such as expensiveness; and physician characteristics, including the physician’s experience and beliefs (Hajjaj et al. 2010). Consumer researchers also note that these decisions involve interactions of task characteristics (consumer medical criteria, preference for prescription in our context) and decision makers’ characteristics and beliefs (belief in intangible benefits and length of experience in our context) (Punj and Stewart 1983). Thus, integrating research on medical decision making with the distinctiveness of life-enhancing products, we propose that three types of nonmedical factors moderate the impact of consumer medical factors on a physician’s decision to prescribe life-enhancing products (see figure 1): consumer characteristics (e.g., consumer preferences), product characteristics (e.g., perceived expensiveness), and physician characteristics (e.g., beliefs, length of experience). This framework parallels the Health Belief Model (HBM), which was developed to explain and predict consumers’ health-related behaviors (Hochbaum 1958). However, there are two key differences between our conceptual framework and HBM. First, we focus on physician decision making, whereas HBM is applied from the consumer’s perspective. Second, our framework includes interactions between medical and nonmedical factors, as well as interactions between the nonmedical factors, whereas HBM does not specify if or how different factors interact, which constitutes one of its major limitations (Glanz, Rimer, and Viswanath 2008).
FIGURE 1.
CONCEPTUAL FRAMEWORK AND HYPOTHESES
Consumer Characteristics
Consumer Preferences. Consumer preference refers to a consumer’s stated preference for a life-enhancing product prescription (vs. exhibiting no stated preference). In the context of traditional medicine, consumers’ preference for a prescription is a leading driver of physicians’ decisions to prescribe a particular product, based on main effects (Kravitz et al. 2005). We expect that for life-enhancing products, consumer preference for a prescription plays an even greater role in the physician’s prescribing behavior, not only by directly increasing the likelihood of a physician writing a prescription but also by weakening the impact of consumer medical criteria.
Why might consumer preferences alter the effect of consumers’ objective medical criteria? The review of medical literature in table 1 indicates that the guidelines for prescribing a life-enhancing product typically are more flexible and less clear than those for traditional therapy products (Allen and Fost 2004). This lack of clarity in prescription guidelines is affirmed by the empirical data collected for this study, where only 4.1% of physicians agreed that “current statements by professional societies provide clear practice guidelines on GH use for healthy but short children.” This flexibility and lack of clarity in guidelines allows more discretion in the physician’s judgments and injects more uncertainty into the physician’s decision. Consumers who are sure about their desire to use the product and prefer a prescription (vs. those who do not have an explicit preference) likely communicate these preferences to their physician. This lowers the uncertainty the physician may experience and in turn lessens the impact of medical criteria on the physician’s likelihood to prescribe the product. Furthermore, stating a preference for a prescription signals the consumer’s desire for autonomy in decision making, making physicians more cognizant of the imposed choice that these consumers face. Botti and Iyengar (2006, 32) note that the paradigm that dominates bioethics in the United States is based on patients’ autonomy and “implicitly states that personal preferences are more important than technical issues in the decision-making process.” When evaluating healthy consumers for a life-enhancing reason, under unclear medical guidelines, physicians may be more likely to act on the autonomy paradigm by making trade-offs, such that they give less weight to medical criteria when consumers prefer a prescription. Thus, we reason that a consumer’s preference for a life-enhancing prescription diminishes the negative impact of consumer medical criteria on the likelihood of prescription (i.e., faster growth rate reduces the likelihood of GH prescription):
H1: Consumer preference for a life-enhancing product prescription weakens the impact of consumer medical criteria on a physician’s likelihood to prescribe.
Product Characteristics
Perceived Product Expensiveness. Given that life-enhancing products do not treat or cure any disease and may come with exorbitant price tags, we also investigate how product expensiveness affects physicians’ decision making. It is well established that product expensiveness (e.g., price) directly decreases product adoption rates and sales (Srinivasan, Vanhuele, and Pauwels 2010); we expect that it also negatively moderates the effect of consumer medical criteria on the physician’s likelihood of prescribing a life-enhancing product. Marketing literature shows that product expensiveness in general reduces product consumption by decreasing a product’s perceived value, consumers’ willingness to buy, and purchase intentions (Dodds, Monroe, and Grewal 1991; Taylor and Bearden 2002).
This effect may be very pronounced for life-enhancing products. These products, especially those based on biological drugs, are extremely expensive. For example, the price of GH for healthy but short children exceeds $52,000 per incremental inch of growth, or $100,000 per child for the duration of the GH treatment (Lee 2006). With approximately 500,000 US children being candidates for GH, the potential cost of treating all of them would be about $50 billion (Silvers et al. 2010). Physicians likely consider these high prices of life-enhancing products in combination with consumer’s medical criteria to make value judgments, as “those who prescribe … treatment must still confront the question whether the benefits of the intervention justify its cost” (Allen and Fost 2004, 650). This consideration is further promoted by the broad, unclear guidelines for prescribing life-enhancing products, which necessitates the use of additional decision criteria by the physician. Therefore, when the product is very expensive (vs. less expensive), we anticipate that a physician is less likely to prescribe a life-enhancing product; that is, product expensiveness strengthens the negative effect of faster growth rate on prescription likelihood.
While traditional therapy drugs typically are covered by insurance and the consumer bears only a portion of the drug price, the cost of most life-enhancing products comes directly out of consumers’ pockets, as these products are elective. In our study context, “access to GH therapy differs depending on the type of insurance coverage. The deep discord between physician recommendations and insurance coverage decisions … represents a major challenge to mechanisms of health care decision-making, access, and costs” (Finkelstein et al. 1998, 663). Thus, in the empirical tests we include price and product affordability to test for the moderating effect of product expensiveness.
H2: Perceived product expensiveness strengthens the impact of consumer medical criteria on a physician’s likelihood to prescribe a life-enhancing product.
Physician Characteristics
Beliefs in Intangible Benefits. Consumers often expect two main types of benefits from using a product: tangible benefits, which are concrete, measurable, and objective, and intangible benefits, which are subjective and harder to measure. For example, a tangible benefit of breast implants might be the improved shape and size of the breast; the intangible benefits could include improved body image, self-confidence, and a more satisfying sex life. Research indicates that 81% of consumers reported increased sex life satisfaction after getting breast implants (McCarthy et al. 2012).
Our framework, however, focuses on the perspective of the physicians. Physicians have their own beliefs about the intangible benefits of life-enhancing products and incorporate them in their prescribing decisions (Maheshwari et al. 2012). For example, some physicians believe that short children have lower self-esteem and poorer quality of life because of their height (Chaplin et al. 2011); others believe that short height has no negative impact on the social, emotional, or behavioral well-being of children or adults (Bullinger 2011). Thus, a physician’s beliefs in the intangible benefits that a life-enhancing product can provide are an important factor that affects the physician’s prescribing decision. The stronger the physician’s belief in improved intangible benefits for the consumer, the more likely that physician is to prescribe a life-enhancing product.
Beyond this main effect, we argue that the physician’s beliefs in the intangible benefits of a life-enhancing product may moderate the impact of (a) the negative main effect of the consumer’s medical criteria on GH prescription and (b) the positive main effect of the consumer’s preference on GH prescription (this main effect is established in the medical literature; thus, we do not formally hypothesize it but do test it empirically). Because of the uncertainty and flexible guidelines for prescribing life-enhancing products (Lee 2006), physicians have substantial discretion in making trade-offs between the importance of medical and nonmedical factors. Even when the guidelines for prescription are clear (e.g., for traditional therapy products), physicians consciously or subconsciously incorporate their own opinions, leading “one to question to what degree physicians are really using EBM [evidence-based medicine] in conjunction with their professional judgement” to reach their final decisions (Hajjaj et al. 2010, 184). Nonmedical factors, including the physician’s belief in the product’s intangible benefits, can change the importance the physician assigns to consumers’ medical criteria or prescription preferences (Hajjaj et al. 2010). In marketing, Stremersch, Landsman, and Venkataraman (2013) find that the impact of a consumer’s preference for a prescription on the physician’s prescribing behavior depends on the patient’s sociodemographics. They conjecture but do not test the notion that this effect might be driven by the physician’s beliefs. In consumer research, Bettman, Luce, and Payne (1998) also show that when faced with multiple goals (in our case, following medical guidelines, meeting consumer needs, using professional beliefs and knowledge), decision makers form constructive preferences characterized by trade-offs. Thus, a physician who believes that a life-enhancing product comes with a host of intangible benefits may give less weight to consumer medical criteria or the consumer’s preference for a prescription. That is, when a strong belief in intangible benefits exists, other factors likely become less important or have less impact on the physician’s prescribing decision. Thus,
H3: A physician’s belief in intangible product benefits weakens the impact of (a) consumer medical criteria and (b) consumer preference for a prescription on the likelihood of prescribing a life-enhancing product.
Physician’s Length of Experience. Another physician characteristic that can impact the decision to prescribe a life-enhancing product is the length of the physician’s experience. Medical literature shows that the length of experience changes physicians’ decision making. For example, less experienced physicians order more tests for patients than their more experienced colleagues (Hajjaj et al. 2010). Marketing research on expertise and medical research on decision making by experienced versus novice physicians also suggest that experience is likely to augment the impact of medical criteria and consumer preference for a prescription on the physician’s prescribing decision (Alba and Hutchinson 1987; Reyna and Lloyd 2006). When experienced physicians make decisions, they consider only a few key factors, whereas novice physicians consider more factors with varying importance (Reyna and Lloyd 2006). Of all the possible factors that might influence the decision to prescribe a life-enhancing product, an experienced physician likely weighs the impact of critical, fundamental factors more (i.e., consumer medical criteria) and discounts the influence of more peripheral factors (e.g., consumer preference for a prescription) (Reyna and Lloyd 2006). Marketing researchers also note that “relative to novices … experts select fewer, but more diagnostic information inputs, and are more consistent when evaluating nonqualified inputs” (Spence and Brucks 1997, 233). More experienced decision makers also appear less susceptible to and place less weight on the opinions of others (Alba and Hutchinson 1987). In line with empirical findings from medical decision-making literature and marketing research on expertise (Reyna 2008; Reyna and Lloyd 2006; Spence and Brucks 1997), we therefore expect that with more experience, physicians rely more on consumers’ medical factors (resulting in a stronger negative main effect of faster growth rate on GH prescription) than on other, nonmedical factors, such as consumer preference for a prescription. We hypothesize:
H4: A physician’s length of experience (a) strengthens the impact of consumer medical criteria but (b) weakens the impact of consumer preference for a prescription on the likelihood of prescribing a life-enhancing product.
METHOD
Research Setting
We tested the hypotheses in the context of prescriptions of GH for healthy but short children. Prior to 2003, GH was used to treat only medical conditions such as Turner syndrome, Prader-Willi syndrome, and chronic renal failure. In 2003, the FDA approved its use in healthy but short children, making it the first biological, also specialty, drug approved for a life-enhancing purpose (i.e., increased height). The FDA approval created a potential annual market for GH worth about $50 billion (Silvers et al. 2010). However, GH can be obtained (legally) only from an endocrinologist, who prescribes and monitors its use, typically in the form of weekly injections over a period of six months to several years.
Research Design
To test our hypotheses, we employ a conjoint study, with experimentally manipulated cases evaluated by a census of US pediatric endocrinologists. To develop the cases and determine the manipulated factors and their appropriate levels, we conducted in-depth interviews in five states with 10 pediatric endocrinologists and consulted the medical literature on GH and FDA guidelines. Medical criteria for evaluating short stature in healthy children include the child’s growth rate, height, and predicted adult height. Review of medical literature (Radovick and McGillivray 2013) and interviews with expert physicians indicated that the child’s growth rate is a critical factor in deciding whether to prescribe GH. While child’s height and predicted adult height are also relevant criteria, they can be somewhat inaccurate (Radovick and McGillivray 2013). Thus, growth rate is the focal consumer medical criterion in our research.
The interviews and a pretest with six physicians also indicated that four cases was the maximum that physicians could evaluate, given the extensive time and effort required. Thus, we followed Lenk et al.’s (1996) recommendation to manipulate variables that we do not predict to interact with one another “within physicians,” using a balanced block design, which resulted in four cases per physician. Each physician was randomly assigned one of 16 combinations, which consisted of four separate patient cases being evaluated for a GH prescription, where (a) the consumer’s preference for prescription, height, predicted adult height, and product price were manipulated within physicians in a balanced 24 factorial incomplete block design (Cochran and Cox 1992; Keppel and Wickens 2004), while (b) the consumer’s growth rate and gender were manipulated between physicians in a 2 × 2 factorial design. We manipulated consumer growth rate between physicians, because of its hypothesized interactive effects as the key focal medical criterion for prescription. Given that we could present only four cases to each physician, we chose not to manipulate the child’s height and predicted adult height “between physicians” in a full factorial design and use them in the analysis as control variables. Consumer gender was manipulated between physicians because the interviews revealed that a within-physician manipulation would bias responses. All manipulated variables featured two levels, low and high, that bracketed the corresponding range of possible values, as indicated in table 2.
TABLE 2.
VARIABLE MANIPULATION AND MEASUREMENT
| Variables | Within / between physicians | Coding/Measures |
|---|---|---|
| Consumer medical criteria | Between | 0 = very slow growth rate (-3SD on the growth curve; 3.2cm/yr; indicates prescription); |
| 1 = slow growth rate (-1 SD on the growth curve; 4.7cm/yr; does not indicate prescription)* | ||
| Consumer's preference for prescription |
Within | 0 = the consumer is neutral about treatment; |
| 1 = consumer wants GH treatment | ||
| Product price | Within | 0 = $9000/yr (price with potential competitors/generics entry); |
| 1 = $22,000/yr (current GH price) | ||
| Product affordability | Measured | What do you think is the typical out-of-pocket expense for GH that families in your practice pay per year? (0 = $0; 100 = $100; 1000 = $1000; 5000 = $5000; 10,000 = $10,000; don't know) |
| Physician's belief in intangible product benefits | Measured | In your opinion, how often does height impair emotional well-being for (1) a child, if the child's height is < 3rd percentile, and (2) emotional well-being for an adult, if the adult's height is < 3rd percentile? (1 = never; 5 = always) |
| Physician's length of experience | Measured | Years in practice (open-ended) |
| Likelihood of prescribing life-enhancing product | Measured | What is the likelihood that you would recommend GH treatment? (1 = very unlikely; 5 = very likely) |
| Control Variables | ||
| Consumer gender | Between | 0 = male; |
| 1 = female | ||
| Consumer current height | Within | 0 = very short (-3SD; 47"/119cm; <1st percentile); |
| 1 = short (-2SD; 49.6"/126cm; 2nd percentile)* | ||
| Consumer predicted adult height | Within | 0 = very short (- 3SD; 5'1"/155cm; <1st percentile); |
| 1 = short (-2SD; 5'4"/163cm; 2nd percentile)* | ||
| Physician gender | Measured | Your gender (0 = male; 1 = female) |
| Physician height | Measured | Height: ____ft. _____in. |
| Physician age | Measured | Year of birth |
| Physician hours in direct patient care | Measured | How many hours per week do you spend in direct patient care? (5 = 1-10hrs; 15.5 = 11-20hrs; 25.5 = 21-30hrs; 35.5 = 31-40hrs; 40 = > 40hrs) |
| Physician number of patients seen weekly | Measured | Please indicate approximately how many children (new and follow-up) you typically see for short stature of any cause weekly? (0 = 0; 3 = 1-5; 8 = 6-10; 13 = 11-15; 16 = ≥16) |
| Gender homophily | N/A | 0 = physician and consumer are of different genders; |
| 1 = physician and consumer are of the same gender | ||
*Numbers presented here are for cases where the consumer is male; numbers used in cases for female consumers are available on request.
Consumer-Related Variables. Consumer medical criteria, growth rate, were manipulated as slow (–1 SD on the growth curve; coded as 1), which parallels the growth curve, or very slow (–3 SD on the growth curve; coded as 0), which corresponds to deviating or “falling off” the growth curve. The manipulation of the consumer preference for a prescription indicated either “the consumer wants GH treatment” (= 1) or “the consumer is neutral about treatment” (= 0). Product price was either $22,000 a year (current price of GH; = 1) or $9,000 (representing market entry of new products or generics; = 0) (Atanu et al. 2006; Ferrándiz 1999). We measured product affordability by asking physicians to indicate the average consumer out-of-pocket expense (co-pay in dollars) for GH in their practice. The control variables—consumer current height and consumer predicted adult height—were set to indicate either short (–2 SD; = 1) or very short height (–3 SD; = 0). All other pertinent factors, such as the consumer’s age, medical and family history, normal lab results, physical exam and birth weight, and lack of coexisting conditions, were held constant (controlled for) in the detailed presentation of each case to the physicians (refer to web appendix A for case examples). These controls indicated that the consumer was healthy, so the only concern was a short stature, rather than a medical disease. Physicians indicated their likelihood of recommending GH for each case, on a five-point scale, from “very unlikely” to “very likely.” Table 2 provides a summary of the manipulations and measures, together with the variable coding.
Physician-Related Variables. In line with literature on the intangible benefits of GH (Chaplin et al. 2011), we measured the physician’s belief in intangible product benefits by asking the physicians to estimate how frequently “height impairs emotional well-being for children” and “height impairs emotional well-being for adults” on a five-point scale, anchored by “never” (1) and “always” (5). The responses to these two questions were significantly correlated (r = .65, p < .001) and were averaged for the analysis. The physician’s length of experience was measured by the number of years in practice. Other control variables included physician gender, height, and age. Gender homophily was coded as 1 if both physician and consumer were of the same gender and 0 otherwise. The questionnaire also included measures of physicians’ practice characteristics and demographics using established procedures (Dillman 2014). It was pretested to ensure clarity and applicability.
Sample. We mailed the instrument in three waves to all qualified members of the Pediatric Endocrine Society (the US society for pediatric endocrinologists) and the endocrine division of the American Academy of Pediatrics (AAP) to reach the universe of US pediatric endocrinologists. To qualify, the pediatric endocrinologist had to be board-eligible or -certified and currently practicing. Those who were in training, were employed by the industry or government, were retired, had participated in survey development, or had unverifiable contact information were excluded. The final sample comprised 727 qualified pediatric endocrinologists in the United States. Endocrinologists who had not treated patients for their short stature within the previous five years were requested to return but not complete the questionnaire. The response rate was 90%, resulting in 656 usable responses. Table 3 contains the descriptive statistics for the study participants.
TABLE 3.
PHYSICIAN DEMOGRAPHICS AND PRACTICE CHARACTERISTICS
| Characteristic | Descriptive statistic |
|---|---|
| Physician Characteristics | |
| Gender (% female) | 46.70% |
| Age | 50.54 ± 10.9 yrs. |
| Years in practice | 17.5 ± 11.1 yrs. |
| Height SD score | 0.06 ± 0.45 |
| Hours per week in direct patient care | 27.14 ± 11.31 hrs. |
| Number of patients seen weekly | 8.29 ± 4.71 |
| Practice Characteristics | |
| Primary base for practice: | |
| Medical school/university | 59.80% |
| Solo/two-person practice | 15.80% |
| Non-university medical group | 10.90% |
| Large multi-specialty group | 13.00% |
| Proportion of time involved in: | |
| Patient care | 69.36% |
| Research | 18.74% |
| Administrative/other | 11.98% |
| Typical insurance coverage in practice: | |
| Private | 61.70% |
| Medicaid | 32.30% |
| Uninsured | 6.00% |
| Office location: | |
| Large metro | 52.30% |
| Small metro/suburban | 43.80% |
| Rural/non-metro | 4.00% |
Analysis
Consistent with Lenk et al.’s (1996) recommendation for analyzing data with a balanced block design, we employed a pooled estimator to obtain consistent, efficient estimates of the hypothesized effects. Specifically, we used a random parameter model with Halton simulations to accommodate the nested structure of the data (four GH prescription decision cases, nested within each physician) and estimated between- and within-physician effects appropriately (Greene 2011). We used a Halton simulation rather than a random draw simulation to estimate the random parameters in the model because this procedure is reported “to reduce the number of draws needed for estimation (by a factor of 90% or more) and reduce the simulation error associated with a given number of draws” (Greene 2012, R621). We also followed Kuhfeld’s simulation procedure in SAS to compute the design efficiency of our study. The results indicate a D-Efficiency of 91.09%, A-Efficiency of 89.0%, and G-Efficiency of 95.1%, in support of the design’s effectiveness for parameter estimation. The equations in the model are as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
where i denotes the physician, j = 1–4 denotes each case, Med = consumer medical criteria, Consumer = consumer’s preference for a prescription, Price = product price, Intg.Belief = physician’s belief in intangible product benefits, Exp = physician’s length of experience, Afford = product affordability, Gend = consumer gender, Hgt = consumer height, Ad.Hgt = consumer predicted adult height, Age = physician age, Ph.Hgt = physician height, Hrs = physician hours in direct patient care, Nmbr = number of patients physician sees weekly, and Homoph= gender homophily. Case-specific variance is represented by εij∼N (0,σ2); both ∼ N (0, σ2) and the βi are random parameters that can change between physicians due to unobserved within-physician heterogeneity. Further, captures between-physician effects, and , , and capture the moderating effects of product affordability, belief in intangible product benefits, and physician’s length of experience, respectively, on the effect of the consumer’s medical criteria on the physician’s likelihood of prescribing a life-enhancing product (hypothesis 2, hypothesis 3a, and hypothesis 4a, respectively). Finally, and capture the moderating effect of the physician’s belief in intangible product benefits and length of experience, respectively, on the impact of the consumer’s preference for prescription on how likely a physician is to prescribe a life-enhancing product (hypothesis 3b and hypothesis 4b).
RESULTS
Model Fit
We compared the hypothesized model against two other models to assess model fit: a model with control variables only and a model without interactions. The log-likelihood ratio tests indicate that the hypothesized model explains significant variance in GH prescription decisions, above and beyond models that contain only control variables (χ216df = 2270.1, p < .001) or include only simple effects (χ26df = 1652.9, p < .001). Better model fit of the hypothesized model is further confirmed by the uniformly lower Akaike information criterion (14975.4 vs. 18878.4 and 16640.3) and Bayesian information criterion (1375 vs. 1972 and 1591.3) than those of the control only and simple effects models, respectively. Thus, the hypothesized model fit the data better than the other two models.
Hypotheses Testing
To clarify the results of the moderation hypotheses, we provide the findings for the nonhypothesized main effect of consumer medical criteria on the likelihood of GH prescription first. Consistent with medical literature, we find that faster-growing children (consumer medical criteria of slow growth rate, which parallels the growth curve) are less likely to get a GH prescription than slower-growing children (consumer medical criteria of very slow growth rate, which deviates from the growth curve) = –.71, p < .001), as table 4 shows. A physician is more likely to prescribe GH even to faster-growing children when consumers prefer a prescription, though, so the consumer’s preference for a prescription weakens the negative impact of the consumer’s medical criteria, in support of hypothesis 1 (= .033, p < .01).
TABLE 4.
RESULTS: DECISION TO PRESCRIBE A LIFE-ENHANCING PRODUCT
| Independent Variables (Manipulation Coding) | Parameter | Parameter Estimate (SE) | |
|---|---|---|---|
| Main effects | |||
| Consumer medical criteria (0 = very slow growth rate; 1=slow growth rate) | γ10 | -.708 (.031)*** | |
| Consumer's preference for prescription (0 = no request; 1=request) | γ20 | .779 (.034)*** | |
| Product price (0 = $9,000; 1 = $22,000) | γ30 | -.077 (.009)*** | |
| Interaction effects | |||
| Consumer medical criteria x Consumer's preference for prescription | H1 (+) | γ40 | .033 (.014)** |
| Consumer medical criteria x Product price | H2 (-) | γ50 | -.034 (.013)*** |
| Consumer medical criteria x Product affordability | H2 (-) | γ11 | -.068 (.003)*** |
| Physician's belief in intangible benefits x Consumer medical criteria | H3a (+) | γ12 | .223 (.009)*** |
| Physician's belief in intangible benefits x Consumer's preference for prescription | H3b (-) | γ21 | -.122 (.010)*** |
| Physician's length of experience x Consumer medical criteria | H4a (-) | γ13 | -.011 (.001)*** |
| Physician's length of experience x Consumer's preference for prescription | H4b (-) | γ22 | -.010 (.001)*** |
| Controls | |||
| Consumer gender (0 = male; 1 = female) | -.141 (.009)*** | ||
| Consumer current height (0 = very short; 1 = short) | -.852 (.006)*** | ||
| Consumer predicted adult height (0 = very short; 1 = short) | -1.428 (.006)*** | ||
| Gender homophily | -.068 (.009)*** | ||
| Physician height | .032 (.008)*** | ||
| Physician age | .010 (.000)*** | ||
| Physician hours in direct patient care | -.008 (.000)*** | ||
| Physician number of patients seen weekly | .035 (.001)*** | ||
*p < .05;
**p < .01;
***p < .001
In support of hypothesis 2, a higher product price strengthens the negative impact of consumer medical criteria on product prescription, so a physician is even less likely to prescribe GH to a faster-growing child, compared with a slower-growing child, when the product price is high (= –.034, p < .001). Similarly, lower product affordability strengthens the negative impact of the consumer medical criteria on the likelihood of the physician’s prescription ( = –.068, p < .001), also in support of hypothesis 2.
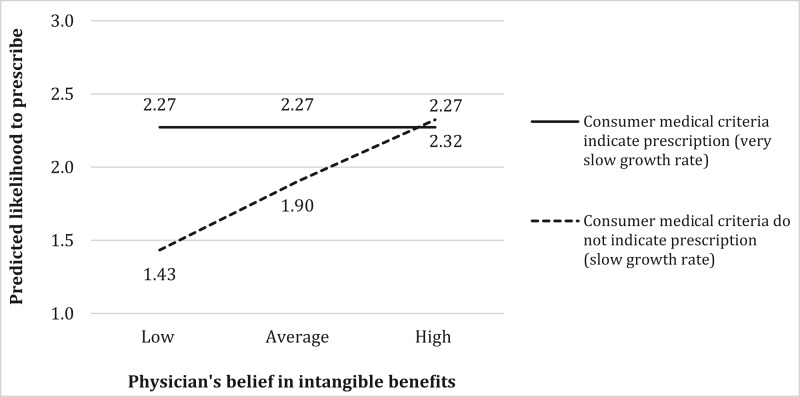
However, if the physician believes in intangible product benefits, she or he is more likely to prescribe GH for faster-growing children, in support of hypothesis 3a (= .22, p < .001). Figure 2 illustrates this interaction by plotting the predicted likelihood of a physician prescribing GH, assuming a short boy (Hgt = 1) with a low predicted adult height (PAH = 1), a consumer who is neutral about a prescription, a low price, high affordability, an experienced physician, and all other control variables at their average values. To further understand this interaction, we followed Spiller et al.’s (2013) method to test the effects of change in the physician’s belief in intangible benefits for different values of medical criteria (i.e., very slow vs. slow growth rate), using a Wald test. The results indicate that for consumers with medical criteria indicating a GH prescription (slower-growing children deviating from the growth curve), the physician’s belief in intangible benefits does not have an effect on the likelihood of prescription (solid line in figure 2). In contrast, for consumers whose medical criteria do not indicate a GH prescription (faster-growing children paralleling the growth curve), increasing the physician’s belief in intangible benefits from low (–2 SD) to high (+2 SD) increases the likelihood of prescription (slope of dotted line coefficient = .89, p < .001). The consumer’s medical criteria also have a significant effect on the physician’s likelihood of prescribing GH when the physician’s belief in intangible product benefits is low (.84, p < .001) but no effect when this belief is high (.05, p > .82).
FIGURE 2.
INTERACTION EFFECT OF PHYSICIAN’S BELIEF IN INTANGIBLE PRODUCT BENEFITS AND CONSUMER MEDICAL CRITERIA
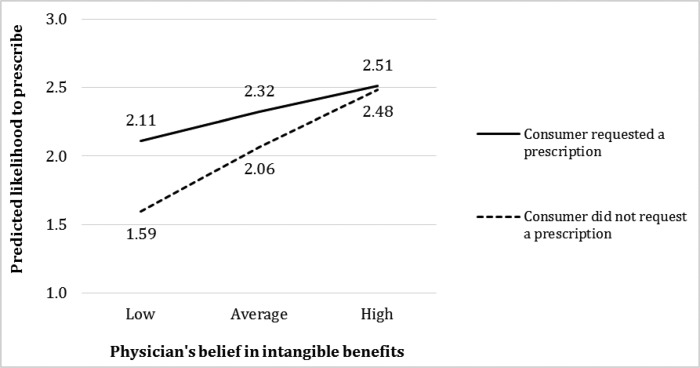
The physician’s belief in intangible product benefits decreases the positive main effect of the consumer’s preference on the likelihood of GH prescription, supporting hypothesis 3b (= –.12, p < .001). Figure 3 illustrates this interaction effect by plotting the predicted likelihood of a physician prescribing GH, assuming a short boy (Hgt = 1) with a low predicted adult height (PAH = 1), medical criteria of slow growth rate, a low price, high affordability, and all other control variables at their average values. A Wald test indicates that an increase in the physician’s belief in the intangible benefits from low (–2 SD) to high (+2 SD) has a stronger effect on the physician’s likelihood of prescribing GH for consumers who did not request a prescription (figure 3, dotted line slope coefficient = .10/.40, p < .01) compared with consumers who did request a prescription (solid line slope coefficient = .22/.89, p < .001). Also, a consumer’s preference for a prescription has a significant effect on a physician’s likelihood of prescribing GH when the physician’s belief in intangible product benefits is low (.52, p < .001) but no effect when this belief in intangible benefits is high (.03, p > .18).
FIGURE 3.
INTERACTION EFFECT OF PHYSICIAN’S BELIEF IN INTANGIBLE PRODUCT BENEFITS AND CONSUMER’S PREFERENCE FOR PRESCRIPTION
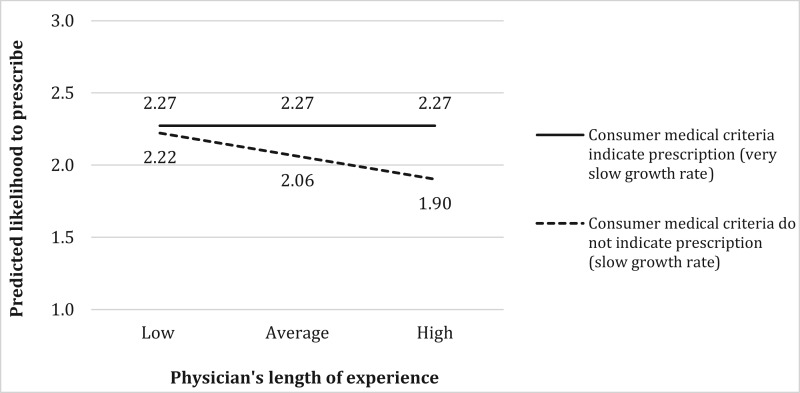
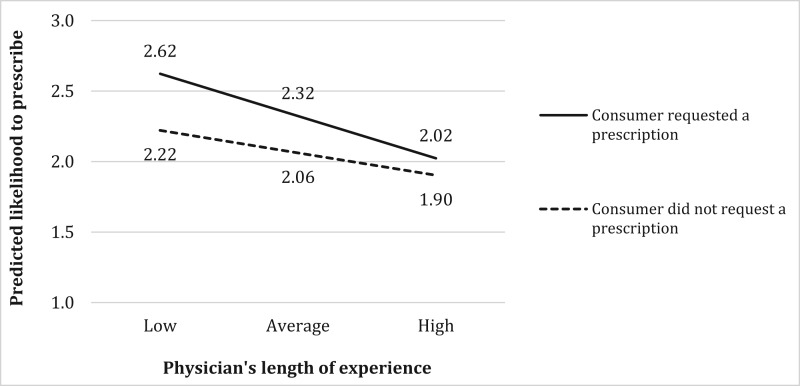
The results show that the length of the physician’s experience plays an important role in the decision to prescribe a life-enhancing product too. Consistent with hypothesis 4a, more experienced physicians assign more weight to consumer’s medical criteria, as indicated by the significant negative interaction (= –.01, p < .001) in the presence of a negative main effect of these medical criteria. Figure 4 illustrates this interaction by plotting the predicted effect for a boy who is short (Hgt = 1), with a low predicted adult height (PAH = 1), when price is low, affordability is high, the consumer is neutral about a prescription, and all other control variables are at average values. A Wald test indicates that when a consumer’s medical criteria indicate a GH prescription (slower-growing children), the length of the physician’s experience does not have an effect on the likelihood of prescribing GH (solid line in figure 4). In contrast, when medical criteria do not indicate a GH prescription (faster-growing children), an increase in the length of the physician’s experience from low (–2 SD) to high (+2 SD) has a significant effect on the likelihood of a GH prescription (dotted line slope coefficient = –.32, p < .001). Thus, a consumer’s medical criteria have a significant effect on how likely an experienced physician is to prescribe GH (.37, p < .001) but no effect among new physicians (.05, p > .82).
FIGURE 4.
INTERACTION EFFECT OF PHYSICIAN’S LENGTH OF EXPERIENCE AND CONSUMER MEDICAL CRITERIA
Finally, consistent with hypothesis 4b, more experienced physicians give less weight to the consumer’s preference for a prescription when deciding whether to prescribe GH than do less experienced physicians (= –.01, p < .001), as illustrated by the plotted predicted effects in figure 5. A Wald test indicates that as the length of the physician’s experience increases from low (–2 SD) to high (+2 SD), it exerts a stronger effect on the likelihood of GH prescription for consumers who requested a prescription (figure 5, slope of solid line coefficient = .60, p < .01) than for consumers who did not make such a request (slope of dotted line coefficient = .32, p < .001). A consumer’s preference for a prescription also has a positive effect on new physicians’ likelihood to prescribe GH (.40, p < .001), but this effect is significantly smaller among experienced physicians (.12, p < .01), representing a 71% decrease.
FIGURE 5.
INTERACTION EFFECT OF PHYSICIAN’S LENGTH OF EXPERIENCE AND CONSUMER’S PREFERENCE FOR PRESCRIPTION
Robustness Checks
We randomly selected 70% of the observations from the original data set as an estimation sample and 30% as a holdout sample to check for out-of-sample predictions (Lenk et al. 1996). The results indicate model prediction accuracy of 95% (error = –.049, SD = .35). We also increased the number of draws in the Halton simulation used to estimate the model from 1,000 to 10,000, which resulted in a .01% change on average in the parameter estimates, indicating that our results are robust. Finally, we excluded the interactions between the nonmedical factors from the analysis and observed that statistical inference about the main findings remains the same; that is, the moderating effect of nonmedical factors on the relationship between consumer medical criteria and prescription likelihood holds.
GENERAL DISCUSSION
The National Science Foundation reports that the “improvement of human performance becomes possible” and, if vigorously pursued, “could achieve a golden age that would be a turning point for human productivity and quality of life” (President’s Council on Bioethics 2003, 29). However, what determines consumers’ access to such improvements? This study proposes a conceptual framework and examines how physicians trade off objective consumer medical criteria with nonmedical, consumer, product, and physician factors before granting consumers access to GH for life-enhancing purposes. We test this framework with a conjoint study of access to GH for healthy but short children, based on a census of US physicians. The results provide insights into why two children with identical medical criteria, such as Josh and Matt in our opening scenario, might experience vastly different outcomes in their access to GH. Specifically, we find that the consumer’s preference for a life-enhancing prescription weakens the impact of objective medical criteria, but product expensiveness strengthens this impact on the likelihood of a product prescription. The stronger the physician’s belief in the intangible product benefits, the weaker the effect of consumer medical criteria and preferences for a prescription on the likelihood of obtaining one. Finally, the length of the physician’s experience plays a moderating role, increasing the impact of medical criteria and decreasing the influence of the consumer’s preference for a prescription on the likelihood that the physician prescribes the product.
Limitations
Like all research, this study has certain limitations. The analysis could have benefited from live cases and actual prescription behavior, instead of case scenarios. However, to ensure high external and internal validity, we developed the case scenarios with extensive input from physicians. Physicians are accustomed to working with, evaluating, and solving patient cases using write-ups and descriptions, as these are extensively used in medical education and training. The participating physicians also provided us with an exhaustive list of factors used to make decisions in their practice, and some of them contributed to the construction and revision of the cases to ensure that they reflected the decision-making process involved in real-life cases. More than 96% of the physicians in our study agreed that the cases reflected their actual prescribing behavior in clinical practice. Furthermore, the research design did not allow for formal testing of process-level mechanisms underlying the hypothesized relationships and findings. Further research might address this limitation with experimental work that identifies the causal explanation for the revealed trade-offs across medical and nonmedical consumer, product, and physician factors in physicians’ decisions to grant access to life-enhancing products. Finally, we were not able to test the effects of three-way interactions (consumer medical criteria × consumer preferences × physician’s belief in intangible benefits; consumer medical criteria × consumer preferences × physician’s length of experience) in our model, because this increased the multicollinearity in the estimation beyond the acceptable variance inflation factor threshold of 10. Given the dummy variable nature of medical criteria and consumer preferences in the analysis, creating instrumental variables for them to address multicollinearity was not feasible. While we did that for physicians’ length of experience and physicians’ belief in intangible benefits (i.e., created an instrument for one of the variables that was orthogonal to the other), it did not sufficiently reduce the multicollinearity to allow for the abovementioned three-way interactions. Future research could design studies to examine boundary conditions for the way consumer preferences for prescriptions moderate the effect of consumer medical criteria on physicians’ decision making.
Research Implications
Our findings provide several implications for research. In particular, we contribute to emerging literature on the consumption of life-enhancing products. Prior consumer research (Riis et al. 2008; Williams and Steffel 2014) addresses issues surrounding consumer perceptions and preferences for life-enhancing product use. However, no studies, to the best of our knowledge, examine factors that influence consumer access to life-enhancing products. We find that several categories of nonmedical factors, ranging from the consumer’s preference for a prescription to product- and physician-related factors, moderate the effect of consumer medical criteria on physicians’ decisions to grant access to life-enhancing products. Physicians also make trade-offs among nonmedical factors, which is a key element of high-quality, rational decision making in consumer research literature (Bettman, Luce, and Payne 1998; Frisch and Clemen 1994). The implications of this research thus align with three categories of nonmedical factors (consumer, product, and physician) that affect physicians’ decision making; we also offer research directions for each category, because our study investigates only a subset of all the potential factors.
In terms of consumer-related factors, we find that consumer medical criteria have less impact on a physician’s decision if the consumer requests a prescription. Previous research on the effects of a consumer’s preference for prescriptions focuses largely on traditional medical products and identifies mostly direct effects on physicians’ prescribing behaviors (Kravitz et al. 2005). In a rare study of interaction effects, Venkataram and Stremersch (2007) find that the consumer’s preference for a prescription for traditional therapy products has more positive effects on prescribing behavior for more effective drugs than for less effective drugs. We extend this research to the domain of life-enhancing products and show that a consumer’s preference for a prescription diminishes the impact of objective consumer medical criteria on prescription decisions. We thus offer evidence of a more “consumerist” decision model (Camacho et al. 2010) and consumer decision empowerment (Camacho, De Jong, and Stremersch 2014) in life-enhancing compared with traditional therapy consumption settings.
An interesting area for future research might be the role of consumers’ motivation (source and nature) to improve themselves beyond their full potential in the decision-making process for life-enhancing products. Also, future research could identify boundary conditions under which consumer preferences for a prescription do not diminish the impact of medical criteria. For example, are there trade-off effects between the level of consumer involvement and the consumption timeframe in consumer and physician decision making for life-enhancing products? Consumer researchers note the lack of insight into how consumers make “high involvement decisions that are laden with attribute trade-offs” (Schaller and Malhotra 2015, 678).
In terms of product-related factors, we find that expensiveness results in a stronger impact of consumer medical criteria on the likelihood of product prescription. Studies show that presenting price information to consumers increases their focus on product functionality (Lee and Zhao 2014). Our study extends this finding to physicians’ decision to grant consumers access to life-enhancing products. Low product affordability and high product prices strengthen the influence of consumer medical criteria (indicating functional or tangible product benefits) on physicians’ prescribing behavior. Therefore, physicians display tendencies similar to consumers’ when trading off between price and product functionality when they decide whether to grant consumers access to life-enhancing products. Future research could investigate other product characteristics, such as the known or unknown side effects of different magnitudes, the convenience or ease of administration, and the presence or nature of competitive products.
In terms of physician-related factors, we isolated the differential moderating effects of two variables: physicians’ belief in the intangible product benefits, and their length of experience. The stronger the physician’s belief in the intangible product benefits, the weaker the effect of consumer medical criteria and preference for prescriptions on the likelihood of obtaining them. This finding indicates that physicians trade off tangible (increased height) for intangible (emotional well-being) benefits when granting consumers access to life-enhancing products, and tangible and intangible benefits are not strictly additive but compensatory (Bettman et al. 1998) in their impact on prescription decisions. The popular press has provided anecdotal evidence of these trade-offs in the case of prescriptions for ADHD (attention deficit hyperactivity disorder) drugs, or “productivity drugs” for highly educated professionals who do not suffer from ADHD but seek to increase their productivity at work for career advancement. Based on interviews with consumers, Schwarz (2015) reports that physicians are more likely to prescribe ADHD medications as productivity drugs, without testing consumer medical criteria to see whether a consumer actually needs an ADHD drug, when the consumer is dressed in a suit, which signals a highly demanding professional position. In this case, physicians’ beliefs about intangible product benefits (i.e., increased productivity demanded of high-level professionals) seemingly moderate and weaken the importance of medical criteria in prescription decisions. Our study provides empirical evidence for this anecdotal finding, showing that physicians’ belief in the intangible product benefits matter the most when the consumer medical criteria do not indicate prescription.
In contrast, the length of a physician’s experience plays a regulating role, increasing the impact of consumer medical criteria and decreasing the influence of the consumer’s preference for a prescription on the likelihood that the physician will prescribe the product. We extend research by Stremersch et al. (2013), which shows that specialists get more requests for prescriptions than primary care physicians but fulfill fewer; we demonstrate that the physician’s expertise positively moderates the impact of consumer medical criteria and negatively moderates the impact of the consumer’s preference on prescription decisions. We also extend consumer behavior literature by showing that expert physicians give more weight to diagnostic information and rely less on the opinions of others (Alba and Hutchinson 1987) when making decisions about consumers’ access to life-enhancing products.
Future research could investigate the role of other physician characteristics that might affect access to life-enhancing products, such as empathy or a relationship with the consumer. Further, physicians’ decision-making process about life-enhancing products is important not only at the prescribing stage but also during the follow-up stage, when they make decisions about continued use. For many life-enhancing products, there is no clear usage endpoint, and the decision to continue using them has important implications for consumers, making this a fruitful avenue for future research. Finally, this study did not directly compare or quantify the differential effects of the hypothesized variables for traditional therapy versus life-enhancing products. Further research might empirically compare the effects of medical versus nonmedical factors in these disparate contexts to draw inferences about unique differences in their effects.
Public Policy Implications
Haberman (2014) has introduced a discussion in the national media about “whether the medical establishment, and perhaps society in general, has gone too far in turning normal conditions, like sadness, into pathologies.” He notes that life-enhancing treatments, originally developed to treat diseases, are now used on “aging bodies enduring normal wear and tear.” Blurry boundaries between what is normal and what is not drive consumers to doctors’ offices, seeking enhancement in many areas of their lives. The strong desire to obtain life-enhancing products might even lead consumers to claim symptoms associated with actual medical diseases. Prescriptions of stimulants such as Ritalin and Adderall to treat ADHD have reached $10 billion in cumulative sales, suggesting that they are being prescribed not just to address the illness but also to enhance cognitive and psychological abilities (IMS Institute 2013). Consumers “chase the American myth of unlimited potential by denying biological limitations, including performance enhancing drugs in sports, searching for self-esteem with cosmetic surgery, or pushing the frontiers of reproductive medicine” (Essig 2013). This is not to imply that only US consumers are striving to obtain perfection. South Korea, for example, has the highest per capita rate of plastic surgeries in the world (Shen 2015). However, there are bound to be cross-cultural differences in consumers’ access and willingness to use life-enhancing products as well as physicians’ prescribing practices due to disparities in cultural and religious values, health care systems, and levels of disposable income across different countries.
The legal basis for prescriptions presumes it is appropriate to limit access for some drugs based on the professional opinion of a qualified physician. The clear intent is to restrict use to conditions only of clinical value. This restriction also may induce underuse—especially from the point of view of the consumer. This research questions the simplistic view of professional and cultural authority (Starr 1982) that underlies such laws. Our finding of direct and indirect effects of the consumer’s preference for prescription on the physician’s decision making points to the potential for overprescribing or overuse, as is the case with antibiotics for viral conditions, when they provide no medical benefit and simply satisfy consumers’ requests, but also result in the development of alarming resistance to antibiotics, which impacts not only a given consumer but society overall. Overprescribing combined with the high prices of life-enhancing products also may create financial burdens on insurance companies, patients, and taxpayers. In the case of GH, “Because of the exorbitant cost and the large number of children … who theoretically qualify for therapy, the use of growth hormone in idiopathic short stature generates questions about the equitable distribution of health care resources and the economic effect on the health care system” (Lee 2006, 2580). Economists project that by 2020, health care spending will be nearly one-fifth of US gross domestic product (Parija 2011).
Further, when dealing with physicians, albeit for life-enhancing purposes, consumers expect definitive answers based on objective information. This research shows that medical criteria have a much weaker impact or no effect when the physician believes in the intangible benefits of the product. This places more burden on the consumer to research and stay informed about any aspect of potential treatments. In our context parents’ desire to increase the height of their short children is understandable, as shorter children might have lower self-esteem (Chaplin et al. 2011). However, there is also evidence that these negative consequences were experienced only by children whose parents found short height to be problematic and sought treatment for it, making their children aware that their height was being regarded as a problem. As Conrad and Potter (2004) note, access to life-enhancing treatments “may perpetuate a sense of inadequacy” (195) and “[s]imply because there is a demand for enhancement and the technology to do it does not mean the result is always beneficial, particularly for society. Issues such as risks, fairness, equity, authenticity and individual choice loom large” (209).
This research illustrates that the medical knowledge of independent experts envisioned in the prescription process alone is not sufficient to overcome consumers’ and physicians’ desires to do more. Beyond the huge cost of treating all potential patients, this study raises questions regarding the inequitable distribution of resources based on consumer advocacy and physicians’ personal beliefs. Consumer education, together with value-based benefit designs (i.e., higher co-pay amounts for less appropriate uses) and reminders in electronic medical records are important tools to address this problem, though they may not be sufficient either. Still, recognition of an inherent desire to do more—especially for children—is the first step toward any rational policy response.
DATA COLLECTION INFORMATION
All data collection was conducted by the first, third, and fourth authors. All analyses were performed by the first and second authors. Data were collected across all US states in 2007–2008.
Supplementary Material
Examples of case scenarios presented to the physicians are provided as a web appendix in the online-only version of the article.
REFERENCES
- Alba Joseph W., Wesley Hutchinson J. (1987), “Dimensions of Consumer Expertise,” Journal of Consumer Research, 13 (4), 411–54. [Google Scholar]
- Allen David B., Fost Norman. (2004), “hGH for Short Stature: Ethical Issues Raised by Expanded Access,” Journal of Pediatrics, 144 (5), 648–52. [DOI] [PubMed] [Google Scholar]
- American Society of Plastic Surgeons, “2013 Plastic Surgery Statistics Report” (2013), https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2013/plastic-surgery-statistics-full-report-2013.pdf.
- Bettman James R., Luce Mary F., Payne John W. (1998), “Constructive Consumer Choice Processes,” Journal of Consumer Research, 25 (3), 187–217. [Google Scholar]
- Botti Simona, Iyengar Sheena S. (2006), “The Dark Side of Choice: When Choice Impairs Social Welfare,” Journal of Public Policy & Marketing, 25 (1), 24–38. [Google Scholar]
- Botti Simona, Orfali Kristina, Iyengar Sheena S. (2009), “Tragic Choices: Autonomy and Emotional Responses in Medical Decisions,” Journal of Consumer Research, 36 (3), 337–52. [Google Scholar]
- Bullinger Monika. (2011), “Psychological Criteria for Treating Children with Idiopathic Short Stature,” Hormone Research in Paediatrics, 76 (suppl 3), 20–23. [DOI] [PubMed] [Google Scholar]
- Camacho Nuno, De Jong Martijn, Stremersch Stefan. (2014), “The Effect of Customer Empowerment on Adherence to Expert Advice,” International Journal of Research in Marketing, 31 (3), 293–308. [Google Scholar]
- Camacho Nuno, Landsman Vardit, Stremersch Stefan. (2010), “The Connected Patient,” in The Connected Customer: The Changing Nature of Consumer and Business Markets, ed. Wuyts Stefan H. K., Dekimpe Marnik G., Gijsbrechts Els, Pieters F. G. M., London: Taylor & Francis, 107–39. [Google Scholar]
- Chaplin John E., Kriström Berit, Jonsson Björn, Hägglöf Bruno, Tuvemo Torsten, Stefan Aronson A., Dahlgren Jovanna, Albertsson-Wikland Kerstin. (2011), “Improvements in Behaviour and Self-Esteem Following Growth Hormone Treatment in Short Prepubertal Children,” Hormone Research in Paediatrics, 75 (4), 291–303. [DOI] [PubMed] [Google Scholar]
- Cochran William G., Cox Gertrude M. (1992), Experimental Designs, New York: Wiley. [Google Scholar]
- Colsman Melissa D., David E Sandberg, Allen David B., Rossi Wilma C. (2005), “Treating Short Stature with Growth Hormone,” http://journalofethics.ama-assn.org/2005/11/ccas2-0511.html. [DOI] [PubMed]
- Conrad Peter, Potter Deborah. (2004), “Human Growth Hormone and the Temptations of Biomedical Enhancement,” Sociology of Health and Illness, 26 (2), 184–215. [DOI] [PubMed] [Google Scholar]
- Dillman Don A. (2014), Internet, Phone, Mail and Mixed-Mode Survyes: The Tailored Design Method, New York: Wiley. [Google Scholar]
- Dodds William B., Monroe Kent B., Grewal Dhruv. (1991), “Effects of Price, Brand, and Store Information on Buyers’ Product Evaluations,” Journal of Marketing Research, 28 (3), 307–19. [Google Scholar]
- Eddy David M. (2005), “Evidence-Based Medicine: A Unified Approach,” Health Affairs, 24 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- Essig Todd. (2013), “When ‘Study Drugs’ Kill (Part 1): How Ambition Becomes Adderall Addiction,” http://www.forbes.com/sites/toddessig/2013/02/10/when-study-drugs-kill-part-1-how-ambition-becomes-adderall-addiction/.
- Ferrándiz Jorge M. (1999), “The Impact of Generic Goods in the Pharmaceutical Industry,” Health Economics, 8 (7), 599–612. [DOI] [PubMed] [Google Scholar]
- Finkelstein Beth S., Silvers J. B., Marrero Ursula, Neuhauser Duncan, Cuttler Leona. (1998), “Insurance Coverage, Physician Recommendations, and Access to Emerging Treatments: Growth Hormone Therapy for Childhood Short Stature,” Journal of the American Medical Association, 279 (9), 663–68. [DOI] [PubMed] [Google Scholar]
- Frisch Deborah, Clemen Robert T. (1994), “Beyond Expected Utility: Rethinking Behavioral Decision Research,” Psychological Bulletin, 116 (1), 46–54. [DOI] [PubMed] [Google Scholar]
- Glanz Karen, Rimer Barbara K., Viswanath Kasisomayajula. (2008), Health Behavior and Health Education: Theory, Research, and Practice, 4th ed., San Francisco: Jossey-Bass, 45–51. [Google Scholar]
- Greene William H. (2011), Econometric Analysis, Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- Greene William H. (2012), LIMDEP Version 10 Econometric Modeling Guide, Plainview, NY: Econometric Software, Inc. [Google Scholar]
- Haberman Clyde. (2014), “Selling Prozac as the Life-Enhancing Cure for Mental Woes,”http://www.nytimes.com/2014/09/22/us/selling-prozac-as-the-life-enhancing-cure-for-mental-woes.html?_r=0.
- Hajjaj Fadi M., Salek M. Sam, Basra Mohammad K. A., Finlay Andrew Y. (2010), “Non-Clinical Influences on Clinical Decision-Making: A Major Challenge to Evidence-Based Practice,” Journal of the Royal Society of Medicine, 103 (5), 178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedén Per, Adams William, Jr.,, Maxwell Patrick, Nava Maurizio, Scheflan Michael, Stan Constantin. (2009), “Aesthetic Breast Surgery: Consulting for the Future—Proposals for Improving Doctor–Patient Interactions,” Aesthetic Plastic Surgery, 33 (3), 388–94. [DOI] [PubMed] [Google Scholar]
- Hitz Felicitas, Ribi Karin, Li Qiyu, Klingbiel Dirk, Cerny Thomas, Koeberle Dieter. (2013), “Predictors of Satisfaction with Treatment Decision, Decision-Making Preferences, and Main Treatment Goals in Patients with Advanced Cancer,” Supportive Care in Cancer, 21 (11), 3085–93. [DOI] [PubMed] [Google Scholar]
- Hochbaum Godfrey M. (1958), “Public Participation in Medical Screening Programs: A Socio-Psychological Study,” PHS Publication No. 572, Washington DC, US Government Printing Office.
- IMS Institute (2013), “Medicine Use and Shifting Costs of Healthcare,” http://www.imshealth.com/en/thought-leadership/quintilesims-institute/reports/use-of-medicines-in-the-us-2013.
- Juengst Eric T. (1997), “Can Enhancement Be Distinguished from Prevention in Genetic Medicine?” Journal of Medicine and Philosophy, 22 (2), 125–42. [DOI] [PubMed] [Google Scholar]
- Keppel Geoffrey, Wickens Thomas D. (2004), Design and Analysis: A Researcher’s Handbook, Upper Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- Kravitz Richard L., Epstein Ronald M., Feldman Mitchell D., Franz Carol E., Azari Rahman, Wilkes Michael S., Hinton Ladson, Franks Peter. (2005), “Influence of Patients’ Requests for Direct-to-Consumer Advertised Antidepressants: A Randomized Controlled Trial,” Journal of the American Medical Association, 293 (16), 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Joyce M., Davis Matthew M., Clark Sarah J., Hofer Timothy P., Kemper Alex R. (2006), “Estimated Cost-Effectiveness of Growth Hormone Therapy for Idiopathic Short Stature,” Archives of Pediatrics and Adolescent Medicine, 160 (3), 263–69. [DOI] [PubMed] [Google Scholar]
- Lee Kelly K., Zhao Min. (2014), “The Effect of Price on Preference Consistency over Time,” Journal of Consumer Research, 41 (1), 109–18. [Google Scholar]
- Lee Mary M. (2006), “Idiopathic Short Stature,” New England Journal of Medicine, 354 (24), 2576–82. [DOI] [PubMed] [Google Scholar]
- Lenk Peter J., DeSarbo Wayne S., Green Paul E., Young Martin R. (1996), “Hierarchical Bayes Conjoint Analysis: Recovery of Partworth Heterogeneity from Reduced Experimental Designs,” Marketing Science, 15 (2), 173–91. [Google Scholar]
- Maheshwari Nidhi, Uli Naveen K., Narasimhan Sumana, Cuttler Leona. (2012), “Idiopathic Short Stature: Decision Making in Growth Hormone Use,” Indian Journal of Pediatrics, 79 (2), 238–43. [DOI] [PubMed] [Google Scholar]
- McCarthy Colleen M., Cano Stefan J., Klassen Anne F., Scott Amie, Van Laeken Nancy, Lennox Peter A., Cordeiro Peter G., Pusic Andrea L. (2012), “The Magnitude of Effect of Cosmetic Breast Augmentation on Patient Satisfaction and Health-Related Quality of Life,” Plastic and Reconstructive Surgery, 130 (1), 218–23. [DOI] [PubMed] [Google Scholar]
- Parija Kavilanz. (2011), “U.S. Will Pay for Half of All Health Care Costs by 2020,” http://money.cnn.com/2011/07/28/news/economy/healthcare_spending_forecast/.
- President’s Council on Bioethics (2003), Beyond Therapy: Biotechnology and the Pursuit of Happiness. Washington, DC: HarperCollins. [Google Scholar]
- Punj Girish N., Stewart David W. (1983), “An Interaction Framework of Consumer Decision Making,” Journal of Consumer Research, 10 (2), 181–96. [Google Scholar]
- Radovick Sally, McGillivray Margaret. (2013), Pediatric Endocrinology: A Practical Clinical Guide, New York: Humana. [Google Scholar]
- Reyna Valerie. (2008), “A Theory of Medical Decision Making and Health: Fuzzy Trace Theory,” Medical Decision Making, 28 (6), 850–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna Valerie, Lloyd Farrell. (2006), “Physician Decision Making and Cardiac Risk: Effects of Knowledge, Risk Perception, Risk Tolerance, and Fuzzy Processing,” Journal of Experimental Psychology: Applied, 12 (3), 179–95. [DOI] [PubMed] [Google Scholar]
- Riis Jason, Simmons Joseph, Goodwin Geoffrey. (2008), “Preferences for Enhancement Pharmaceuticals: The Reluctance to Enhance Fundamental Traits,” Journal of Consumer Research, 35 (3), 495–508. [Google Scholar]
- Rosenthal Meredith B., Berndt Ernst R., Donohue Julie M., Frank Richard G., Epstein Arnold M. (2002), “Promotion of Prescription Drugs to Consumers,” New England Journal of Medicine, 346 (7), 498–505. [DOI] [PubMed] [Google Scholar]
- Saha Atanu, Grabowski Henry, Birnbaum Howard, Greenberg Paul, Bizan Oded. (2006), “Generic Competition in the US Pharmaceutical Industry,” International Journal of the Economics of Business, 13 (1), 15–38. [Google Scholar]
- Schaller Tracey King, Malhotra Naresh K. (2015), “Affective and Cognitive Components of Attitudes in High-Stakes Decisions: An Application of the Theory of Planned Behavior to Hormone Replacement Therapy Use,” Psychology & Marketing, 32 (6), 678–95. [Google Scholar]
- Schwarz Alan. (2015), “Workers Seeking Productivity in a Pill Are Abusing A.D.H.D. Drugs,” New York Times, April 19, A1.
- Shen Lucinda. (2015), “Here Are the Vainest Countries in the World,” http://www.businessinsider.com/here-are-the-vainest-countries-in-the-world-2015-8.
- Silvers J. B., Marinova Detelina, Mercer Mary B., Connors Alfred, Cuttler Leona. (2010), “A National Study of Physician Recommendations to Initiate and Discontinue Growth Hormone for Short Stature,” Pediatrics, 126 (3), 468–76. [DOI] [PubMed] [Google Scholar]
- Spence Mark T., Brucks Merrie. (1997), “The Moderating Effects of Problem Characteristics on Experts’ and Novices’ Judgments,” Journal of Marketing Research, 34 (2), 233–47. [Google Scholar]
- Spiller Stephen A., Fitzsimons Gavan J., Lynch John G., Jr.,, McClelland Gary H. (2013), “Spotlights, Floodlights, and the Magic Number Zero: Simple Effects Tests in Moderated Regression,” Journal of Marketing Research, 50 (2), 277–88. [Google Scholar]
- Srinivasan Shuba, Vanhuele Marc, Pauwels Koen. (2010), “Mind-Set Metrics in Market Response Models: An Integrative Approach,” Journal of Marketing Research, 47 (4), 672–84. [Google Scholar]
- Starr Paul. (1982), The Social Transformation of American Medicine, New York: Basic Books. [Google Scholar]
- Stremersch Stefan, Landsman Vardit, Venkataraman Sriram. (2013), “The Relationship between DTCA, Drug Requests, and Prescriptions: Uncovering Variation in Specialty and Space,” Marketing Science, 32 (1), 89–110. [Google Scholar]
- Taylor Valerie, Bearden William. (2002), “The Effects of Price on Brand Extension Evaluations: The Moderating Role of Extension Similarity,” Journal of the Academy of Marketing Science, 30 (2), 131–40. [Google Scholar]
- United Nations Office on Drugs and Crime, “World Drug Report” (2012), www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_small.pdf.
- Venkataraman Sriram, Stremersch Stefan. (2007), “The Debate on Influencing Doctors’ Decisions: Are Drug Characteristics the Missing Link?” Management Science, 53 (11), 1688–701. [Google Scholar]
- Weintraub Arlene, Arndt Michael. (2005), “My, How You’ve Grown,” http://www.bloomberg.com/bw/stories/2005-11-27/my-how-youve-grown.
- Williams Elanor F., Steffel Mary. (2014), “Double Standards in the Use of Enhancing Products by Self and Others,” Journal of Consumer Research, 41 (2), 506–25. [Google Scholar]
- World Health Organization (2008), “Where Are the Patients in Decision-Making about Their Own Care?” http://www.who.int/management/general/decisionmaking/WhereArePatientsinDecisionMaking.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.