Abstract
Objective:
The study aims at the exploration of calcium oxalate (CaOx) crystal growth inhibition potential of Cynodon dactylon, Emblica officinalis, Kalanchoe pinnata, and Bambusa nutans ethyl acetate fraction rich in polyphenol and flavonoid.
Materials and Methods:
Ethyl acetate fraction was separated from the hydromethanolic extract of C. dactylon, E. officinalis, K. pinnata, and B. nutans followed by quantitative analysis for total polyphenol and flavonoid content. Ethyl acetate fraction of all the plants were subjected to in vitro screening for the inhibition of CaOx crystals growth induced by sodium oxalate.
Results:
The results signify rich presence of polyphenols and flavonoids in K. pinnata and E. officinalis ethyl acetate fractions followed by C. dactylon and B. nutans. Ethyl acetate fractions of B. nutans shoot, E. officinalis fruit, and K. pinnata leaf have excellent in vitro CaOx crystal growth inhibition potential based on both the comparative concentration and the time level to achieve IC50.
Conclusion:
The study outcome substantiates potential in vitro CaOx crystal dissolution and crystal growth inhibition properties of E. officinalis, B. nutans, C. dactylon, and K. pinnata. Rich presence of caffeic acid, ferulic acid, and luteolin in ethyl acetate fraction of B. nutans leaf, and chebulinic acid, chebulagic acid, gallic acid, ellagic acid, and quercetin of E. officinalis may have produced prominent crystal aggregation inhibition response.
KEYWORDS: Antilithiatic, Bambusa nutans, calcium oxalate, Cynodon dactylon, Emblica officinalis, flavonoid, Kalanchoe pinnata, polyphenol
INTRODUCTION
In vitro calcium oxalate (CaOx) salt crystallization study is of elementary importance for urolithiasis potential analysis of drugs. Various alternative strategies are also being used by the researchers that differ qualitatively and quantitatively within the extent that they must possibly reproduce all the aspects of the crystallizing activity occurring in the excretory organ system. No matter what system is used to study crystallization modulating potential of a drug, the target is to measure three key aspects: first, the chemistry concerning prevailing supersaturation issue in the kidney; second, different processes that might interfere throughout the course of the crystallization; and third, how close is the process that the experimenter is trying to mimic compared to what actually happens in the biological atmosphere.
Sequential steps of stone formation, from crystal nucleation induction to the final clinically complicated stone formation, are modulated by mutual balance between promoters and inhibitors [Figure 1]. A gentle state supersaturation can probably act as a good representative of the intrarenal scenario than a system that decays the equilibrium status. The constant composition technique and the mixed suspension product removal techniques can closely resemble the gentle supersaturation process of kidney.[1] Crystallization strategies used in urolithiasis drug analysis are of several forms, that is, in vivo, in vitro, or in the presence of cultured cells, and they could be objective, subjective, or semiquantitative. The processes can involve the utilization of complicated pricey instruments or could be straightforward and low cost. The key to assess the crystallization behavior interference potential of a drug is to explore the target matching with a suitable in vitro crystallization-related experimental procedure.[2,3] This proposed study protocol involves a quantitative in vitro process to find out the interference level of the plant-derived enriched extracts on sodium oxalate–induced in vitro CaOx crystallization process.
Figure 1.
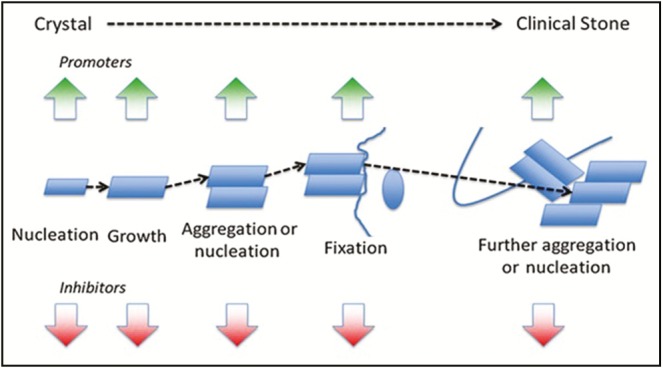
Sequential schema of stone development from nucleation of crystal to a clinical stone formation
MATERIALS AND METHODS
Collection and identification of plant
Cynodon dactylon whole plant, Emblica officinalis fruit, Kalanchoe pinnata leaf, and Bambusa nutans shoot were collected between October 2014 and April 2015 from Rewa, Raisen, and Bhopal districts of Madhya Pradesh, India. Herbarium was prepared for all the four plants and authenticated by Dr. Zia Ul Hasan, Professor and Head, Herbarium Department, Department of Botany, Saifia College of Science Bhopal, Madhya Pradesh. Voucher specimen no. 456/Bot/saifia/14 was allotted. The plant material was made completely clean, dust free, and dried under the shade for 15–30 days. Dried plant parts were pulverized with a mechanical pulverizer to form coarse powder, and stored in airtight container for further studies.
Extraction of plant material
The coarse powder was defatted with petroleum ether (40°C–60°C) for 72h using Soxhlet apparatus separately for individual plant material; the extract was filtered and marc dried. Dried marc was further extracted with methanol/water (50:50) for 72h using Soxhlet apparatus, extract was filtered, and one-third of the solvent was removed by evaporation below 50°C. The remaining solvent was completely evaporated up to a constant weight with rotary vacuum evaporator (Superfit, India) at 50°C. The dried extracts were weighed; percentage yield calculated; and color, consistency, and appearance of all the extracts were noted.[4]
The dry hydromethanolic extract was individually added with 25 times of 1N hydrochloric acid and maintained at 60°C for 30min and further at room temperature for 2h. The extract was filtered, ethyl acetate was added, and the supernatant ethyl acetate phase was separated. The process was repeated thrice, all the supernatant phase was pooled and completely evaporated.[5] The extracts were then weighed, percent yield calculated, and phytochemical test for the presence of flavonoid, tannin, and phenolic compounds was performed.
Quantitative analysis of ethyl acetate fraction
The ethyl acetate fraction of C. dactylon, E. officinalis, K. pinnata, and B. nutans was subjected to qualitative analysis for the estimation of total flavonoids and polyphenolic compounds.
Estimation of total polyphenol
The total phenolic content of the ethyl acetate fraction was determined according to the Folin–Ciocalteu method.[6] This colorimetric method is based on the reduction of a phosphotungstate/phosphomolybdate complex by phenolics to form blue-colored reaction products in alkaline conditions. Ethyl acetate fraction (0.5mL) (20 µg/mL in methanol) was added with 250 µL of Folin–Ciocalteu reagent and 1.25mL of 20% aqueous sodium carbonate solution. After incubation for 45min in dark, the absorbance of this reaction mixture was spectrophotometrically (double beam ultraviolet/visible spectrophotometer; EI 1372, Japan) recorded at 725nm against a blank (0.5mL of extract was replaced by 0.5mL of extracting solvent). Total phenolic content was determined in comparison with the standard gallic acid equivalent (µg/mg of dry mass), which is a common reference compound.[7]
Estimation of total flavonoids
Aluminum chloride colorimetric technique was used for the estimation of flavonoids following the method of Siddique et al.[8] Ethyl acetate fraction (0.5mL) (25 µg/mL in methanol) was mixed with 0.1mL of 10% aluminum chloride, 0.1mL of 1M potassium acetate, and 2.8mL of distilled water. The reaction mixture was kept at room temperature for 30min, and the absorbance was spectrophotometrically measured at 415nm. Standard quercetin calibration curve was plotted by preparing the quercetin standard solutions at 10–70 µg/mL concentrations in methanol. The amount of flavonoid was calculated from the standard graph of quercetin.
In vitro inhibition of CaOx crystals growth induced by sodium oxalate
To assess the antilithiatic potential of plants under investigation, in vitro oxalate crystallization–inhibiting capacity was studied. This model investigated the crystallization of CaOx crystals from aqueous solution in the presence or absence of ethyl acetate fraction of C. dactylon, E. officinalis, K. pinnata, and B. nutans. The solution of calcium chloride and sodium oxalate was prepared in distilled water at the concentration of 4 mmol/L separately in Tris buffer (0.05mol/L) containing 0.15mol/L of sodium chloride at pH 6.5. Ethyl acetate fraction of each plant was dissolved in methanol at 5, 10, 25, 50, and 100mg/mL concentrations. Calcium chloride solution (9.5mL) was mixed with 1mL of ethyl acetate fraction at different concentrations (5–100mg/mL). Crystallization was started by adding 9.5mL of sodium oxalate solution and vigorous stirring for 1min. The mixture was then covered with a glass plate and set aside for crystallization at 30°C temperature. The optical density of the solution was monitored at 620nm using a spectrophotometer at different time intervals. The rate of sedimentary crystal formation was estimated by comparing the absorbance value in the presence of ethyl acetate fraction with that of control.[9,10] The percentage inhibition of crystal formation was calculated using the following formula:
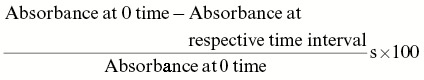
Result
Extractive values
Percentage yield of hydromethanolic extract was 2.85%, 20.00%, 5.52%, and 12.74%, respectively, for C. dactylon, E. officinalis, K. pinnata, and B. nutans. Ethyl acetate fraction yield was obtained as 0.47%, 0.28%, 0.52%, and 0.02% for C. dactylon, E. officinalis, K. pinnata, and B. nutans from the respective hydromethanolic extracts. Qualitative analysis of the hydromethanolic extract of C. dactylon showed a rich presence of carbohydrates, flavonoids, proteins and amino acids, tannins, and phenolic compounds. The presence of alkaloids and glycosides was found to be less. The hydromethanolic extract of E. officinalis showed a rich presence of flavonoids, tannins, and phenolic compounds and a poor presence of glycoside, whereas that of K. pinnata showed a rich presence of carbohydrate, flavonoids, tannins, and phenolic compounds and a poor presence of alkaloids and glycosides. The hydromethanolic extract of B. nutans extract showed the presence of carbohydrates, flavonoids, tannins, and phenolic compounds. The ethyl acetate fraction of C. dactylon, E. officinalis, K. pinnata, and B. nutans was subjected to quantitative estimation of total flavonoid and phenolic compounds. The content of total flavonoid was 40.00%, 53.75%, 65.00%, and 5.25%, whereas total polyphenol was 46.83%, 66.37%, 72.39%, and 47.56%, respectively, in the ethyl acetate fractions of C. dactylon, E. officinalis, K. pinnata, and B. nutans.
Inhibition of CaOx crystal formation
The ethyl acetate fraction of C. dactylon at 100mg/mL concentration showed above 50% inhibition (IC50) of CaOx crystal formation after 90 and 120min of time interval. In case of ethyl acetate fraction of E. officinalis, above IC50 crystal growth inhibition was observed at more than 25mg/mL concentration after 90 and 120min interval. The ethyl acetate fraction of K. pinnata at and above 50mg/mL concentration showed more than 50% inhibition of crystal formation after 30min. The ethyl acetate fraction of B. nutans showed IC50 crystal formation inhibition at above 25mg/mL concentration after 60min interval. The ethyl acetate fractions of E. officinalis and B. nutans had shown above 50% inhibition in the rate of crystal formation at 25mg/mL concentration, respectively, after 90 and 60min of incubation whereas C. dactylon and K. pinnata ethyl acetate fractions showed above 50% crystal growth inhibition at 100 and 50mg/mL concentration, respectively, after 90 and 30min of incubation [Figures 2–5].
Figure 2.
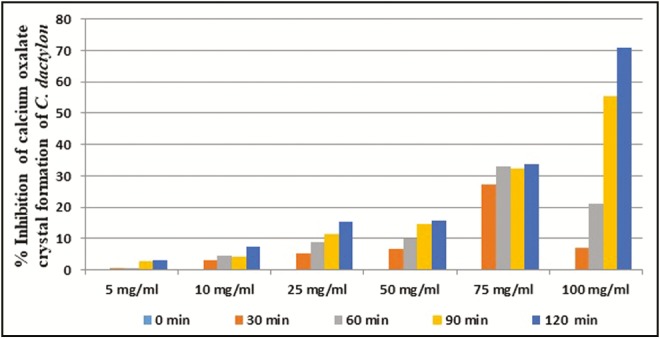
In vitro percentage inhibition of calcium oxalate crystal formation by C. dactylon ethyl acetate fraction
Figure 5.
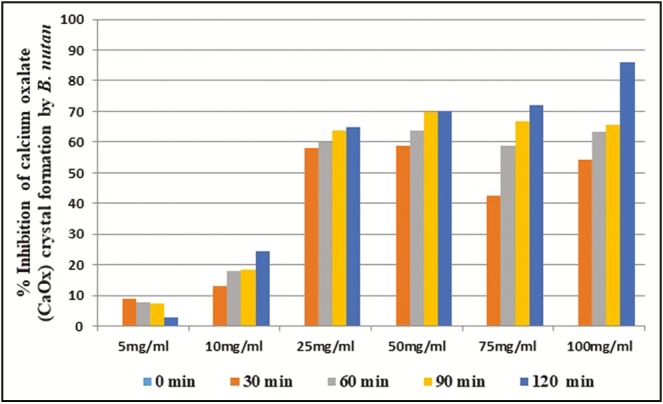
In vitro percentage inhibition of calcium oxalate crystal formation by B. nutans ethyl acetate fraction
Figure 3.
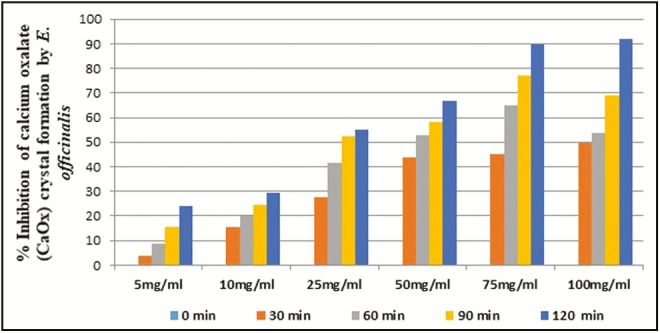
In vitro percentage inhibition of calcium oxalate crystal formation by E. officinalis ethyl acetate fraction
Figure 4.
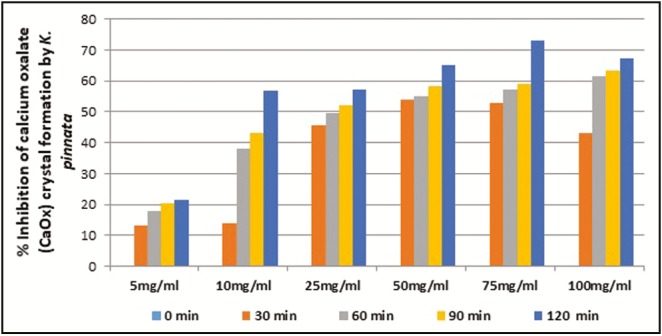
In vitro percentage inhibition of calcium oxalate crystal formation by K. pinnata ethyl acetate fraction
The ethyl acetate fractions of C. dactylon, E. officinalis, K. pinnata, and B. nutans showed excellent in vitro inhibition of CaOx crystal formation, that is, 70.88%, 92.02%, 67.15%, and 86.09%, respectively, in 100mg/mL concentration after 120min of exposure. B. nutans, E. officinalis and K. pinnata have achieved IC50 within 30min of incubation at 100mg/mL concentration, whereas C. dactylon required 60min to achieve IC50 [Figure 6]. The results signify that ethyl acetate fractions of B. nutans shoot, E. officinalis fruit, and K. pinnata leaf have excellent in vitro CaOx crystal growth inhibition potential based on both the comparative concentration and time level to achieve IC50.
Figure 6.
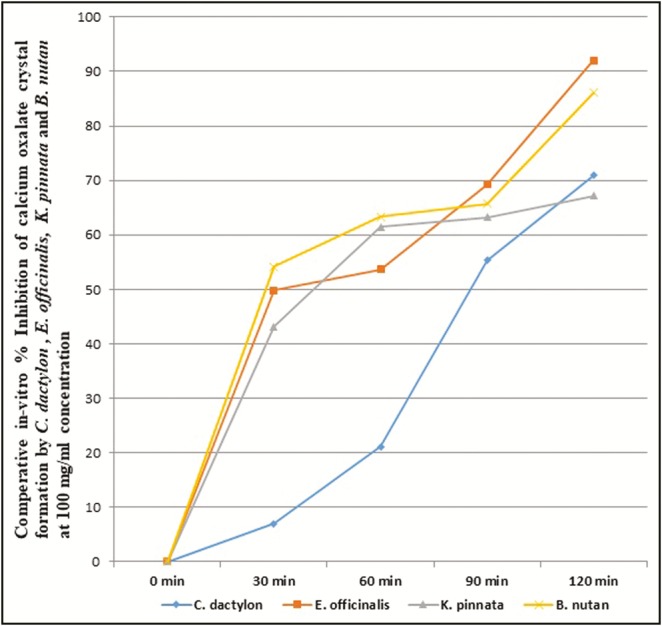
Comparative in vitro percentage inhibition of calcium oxalate crystal formation by C. dactylon, E. officinalis, K. pinnata, and B. nutans ethyl acetate fraction at 100mg/mL concentration
DISCUSSION
Nephrolithiasis is a commonly found pathological condition all over the world, exploiting approximately 20% of the adult population. Formation of crystals in the urinary tract is driven by urinary supersaturation, which is the primary requisition for large stone formation in subsequent stages, though all crystal initiation do not essentially cause stone formation.[3,11] CaOx urinary stones are the most common of all kinds of urinary organ stones. Researchers have shown that crystal retention is an essential step for the formation of clinically symptomatic stone. Crystal agglomeration has long been recognized as the most vital process resulting in crystal retention. In the patients with repeated calcium stone occurrence, it is conjointly prompted by the reduced ability of excretion.[12,13] Cellular injury potentiates CaOx crystal formation and interaction within renal epithelial cells. Salts or CaOx crystals play a major role in the formation, retention, and development of stones.[14] Study of in vitro CaOx crystal dissolution and inhibition is of high value to assess how it differs from the conventional in vivo dissolution, as in the case of in vitro dissolution, the urinary crystal is prompted to dissolve in an appropriate ideal solvent. In vitro dissolution is achieved in a condition where a constant supply of nutrients is maintained for the expansion of solubility. This kind of condition, which is principally desired in vivo to dissolve the stones is nearly impossible to maintain. When the dissolution of stone cannot be achieved, it is commonly preferred to use the growth inhibitors. Many chemical compounds, herbal extracts, and fruit juices can act as growth inhibitors, and they are being assessed on in vitro and in vivo studies.[15] Supersaturation of urine with CaOx is the foremost important aspect of urinary stone formation, along with other factors such as crystallization, nucleation, growth, and aggregation.[16,17] Therefore, if supersaturation or in later steps crystallization is prevented, then pathology is ought to be avoided. In clinical practice also, measures are typically adapted to scale back supersaturation in urinary track, e.g., increasing fluid intake and dietary modification along with medical care.
The under trial herbal remedies C. dactylon, E. officinalis, K. pinnata, and B. nutans are evidence-based handpicked ones, which are selected for pilot in vitro study to explore the possible phytopharmacological correlation based on the fundamentals of reverse pharmacology. Reverse pharmacology is outlined as a transdisciplinary that initiates drug discovery and development from the information of traditional uses following experimental studies. The trial of reverse pharmacology is cost-effective and time-saving in contrast to the long and costly random screening of herbs for drug development.[18] This has enabled the researchers to deal with the pharmacotherapy of urinary stone with the alternative treatments as not many herbal drugs are available to treated nephrolithiasis.[19] The results showed that K. pinnata had the highest content of total flavonoid and polyphenol followed by E. officinalis and C. dactylon. Though B. nutans had lowest flavonoid content, its polyphenol content was equivalent to C. dactylon. The results showed excellent in vitro CaOx crystal growth inhibition potential of B. nutans shoot, E. officinalis fruit, and K. pinnata leaf ethyl acetate fraction based on the comparative concentration level and time extent to achieve IC50. On the basis of maximum in vitro CaOx crystal formation inhibition ability at 100mg/mL concentration after 120min of exposure, the ethyl acetate fractions can be arranged in a descending order as E. officinalis, B. nutans, C. dactylon, and K. pinnata.
Acid-hydrolyzation of apple juice with moushmi (Citrus medica) and amla (E. officinalis) enhances solubility of renal stones of different forms (whole and powdered) compared to non-hydrolyzed juice.[20] Consumption of hot water extract of B. nutans shoot daily for 7 days is helpful in the reduction of urinary calculus.[3] The alcoholic extract of leaves of B. vulgaris showed in vitro inhibitory effect on the formation of CaOx precipitate and crystallization.[21] Contemporary juice extracted from the leaves of Bryophyllum pinnatum was administered to patients having stones. Regular intake of the juice effectively dissolved the stones in spite of its position, nature, and former treatments. It additionally expedited decreased oxalate excretion with increased citrate excretion suggesting antilithiatic properties.[22] Polyherbal tablets containing K. pinnata extracts as one of the constituents were found effective against the accumulation of CaOx crystals and prevention of stone formation within the kidneys.[23] C. dactylon extracts, i.e., n-butanol fraction and n-butanol phase remnant, given with drinking water showed beneficial effects on the prevention and elimination of ethylene glycol–induced kidney calculi in rats.[24]
Phytochemical evaluation showed the presence of phenolic compounds, flavonoids, and tannins in all the four plants. Flavonoids and polyphenolic compounds have diverse pharmacological effects such as anticancer, antioxidant, antiaging, and antibacterial.[25] Flavonoids can prevent adhesion of CaOx crystals in urinary track by virtue of free radical scavenging effects and stop further injury in the process of kidney stone formation.[26,27] Total flavonoid content of Desmodium styracifolium, a Chinese herbal medicine;[28] honeybee product called propolis composed of phenolic, terpenes, essential oils, and especially flavonoids;[29] and avocado leaf (Persea americana Mill.) extract with high flavonoid content have been reported to have antilithiasis activity.[30] Flavonoid diosmin (10 and 20mg/kg) had shown very good antiurolithiatic activity similar to cystone on ethylene glycol and ammonium chloride–induced urolithiasis in experimental rats.[31] Polyphenols from grape seed extract prevented CaOx monohydrate–induced papillary calculus formation,[32] and Piper nigrum polyphenol extract inhibited sodium oxalate–induced oxidative stress in rat kidney.[33] Procyanidin phenolic compounds isolated from Quercus gilva Blume were effective on acute urolithiasis in the experimental animal. Dietary polyphenol caffeic acid had a preventive effect on ethylene glycol–induced kidney stones in rats.[34] Effect of vanillic acid on infectious urolithiasis caused by urease-producing bacteria was comparable with that of other known struvite/apatite crystallization inhibitors (acetohydroxamic acid, pyrophosphate) with a strong inhibition of bacterial growth and the formation of crystals.[35] Procyanidin, vanillic acid, rutin, and curcumin like polyphenolic compounds are known to have antioxidant and anti-inflammatory activities, which may contribute to their antiurolithiatic effect.[36]
CONCLUSION
This study outcome substantiates that E. officinalis, B. nutans, C. dactylon, and K. pinnata have potential in vitro CaOx crystal dissolution and crystal growth inhibition properties. Phytochemical analysis revealed highest flavonoid and polyphenol content in K. pinnata and lowest flavonoid content in B. nutans, though it has good polyphenol content. Study showed excellent CaOx crystal growth inhibition potential of B. nutans shoot followed by E. officinalis fruit and K. pinnata leaf. This may be due to difference in the presence of a particular flavonoid or polyphenol compound signifying a high-level presence of one or more polyphenolic constituents in B. nutans. The ethyl acetate fraction of B. nutans leaf is reported to contain seven phenolic acids, that is, caffeic acid, p-coumaric acid, sinapic acid, ferulic acid, coumaroylquinic acid, 5-feruloyquinic acid, and dihydroxybenzoic acid. Caffeic acid along with flavonoid homoorientin, luteolin, and ferulic acid were detected in high quantity in the ethyl acetate fraction of B. nutans.[37] Rich synergistic presence of caffeic and ferulic acid may have produced the prominent crystal aggregation inhibition response of B. nutans. Amla (E. officinalis) is highly valued in traditional Indian medicine containing tannins, flavonoids, phenolic compounds, saponins, terpenoids, especially chebulinic acid, chebulagic acid, emblicanin, gallic acid, ellagic acid, and quercetin.[38] Bogucka-Kocka et al.[39] reported the presence of rich quantity of ferulic and caffeic acid in K. pinnata leaf extracts along with antioxidant and cytotoxic activities. Further evaluations of these plants are being carried out in animal models to explore the utility potential in urolithiasis treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kavanagh JP. In vitro calcium oxalate crystallisation methods. Urol Res. 2006;34:139–45. doi: 10.1007/s00240-005-0027-z. [DOI] [PubMed] [Google Scholar]
- 2.Hess B, Jordi S, Zipperle L, Ettinger E, Giovanoli R. Citrate determines calcium oxalate crystallization kinetics and crystal morphology studies in the presence of Tamm–Horsfall protein of a healthy subject and a severely recurrent calcium stone former. Nephrol Dial Transplant. 2000;15:366–74. doi: 10.1093/ndt/15.3.366. [DOI] [PubMed] [Google Scholar]
- 3.Hess B, Meinhardt U, Zipperle L, Giovanoli R, Jaeger P. Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res. 1995;23:231–8. doi: 10.1007/BF00393304. [DOI] [PubMed] [Google Scholar]
- 4.Harborne JB. Phytochemical methods: a guide to modern technique of plant analysis. London, UK: Chapman and Hall; 1998. [Google Scholar]
- 5.Shabi MM, Gayathri K, Venkatalakshmi R, Sasikala C. Chemical constituents of hydroalcoholic extract and phenolic fraction of Cynodon dactylon. Int J ChemTech Res. 2010;2:149–54. [Google Scholar]
- 6.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–92. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 7.Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enology Viticulture. 1997;28:49–55. [Google Scholar]
- 8.Siddique NA, Mujeeb M, Najmi AK, Mohd A. Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. African J Plant Sci. 2010;4:1–5. [Google Scholar]
- 9.Pachana A, Wattanakornsri A, Nanuam J. Application of small caltrops (Tribulus terrestis) to inhibit calcium oxalate monohydrate crystallization. Sci Asia. 2010;36:165–8. [Google Scholar]
- 10.Agrawal K, Varma R. Investigation antiurolithiatic potential of Phyllanthus niruri L. a member of the family Euphorbiaceae. Ame J Phytomed Clin Therap. 2014;2:423–31. [Google Scholar]
- 11.Kok DJ. Intratubular crystallization events. World J Urol. 1997;15:219–28. doi: 10.1007/BF01367659. [DOI] [PubMed] [Google Scholar]
- 12.Robertson WG, Peacock M, Nordin BE. Calcium crystalluria in recurrent renal-stone formers. Lancet. 1969;2:21–4. doi: 10.1016/s0140-6736(69)92598-7. [DOI] [PubMed] [Google Scholar]
- 13.Finlayson B, Reid F. The expectation of free and fixed particles in urinary stone disease. Invest Urol. 1978;15:442–8. [PubMed] [Google Scholar]
- 14.Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 15.Vaidya AD, Devasagayam TP. Current status of herbal drugs in India: an overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlayson B. Symposium on renal lithiasis. Renal lithiasis in review. Urol Clin North Am. 1974;1:181–212. [PubMed] [Google Scholar]
- 17.Khan SR. Structure and development of calcium urinary stones. In: Bonucci E, editor. Calcification in biological systems. Boca Raton, FL: CRC Press; 1992. pp. 345–63. [Google Scholar]
- 18.Vaidya ADB. Reverse pharmacological correlates of Ayurvedic drug actions. Ind J Pharmacol. 2006;38:311–5. [Google Scholar]
- 19.Vaidya ADB. Ayurveda in transition. Vol 1. Kottakkal, Kerala: Arya Vaidya Sala; 2010. Reverse pharmacology a paradigm shift for new drug discovery based on Ayurvedic epistemology; pp. 27–38. [Google Scholar]
- 20.Sinha MR, Tagore RN. Effect of acid-hydrolyzation of fruits juice of apple (Malus domestica), moushmi (Citrus medica) and amla (Emblica officinalis) on solubility of urinary stones. Asian J Chem. 2010;22:4840–6. [Google Scholar]
- 21.Garg A, Singh V, Shukla A, Jain CP, Pandey P. Evaluation of inhibition effect of alcoholic extract from Bambusa vulgaris leaves on calcium oxalate crystallization: in vitro study. Asian J Biomaterial Res. 2016;2:91–3. [Google Scholar]
- 22.Gahlaut A, Pawar SD, Mandal TK, Dabur R. Evaluation of clinical efficacy of Bryophyllum pinnatum Salisb for treatment of lithiasis. Int J Pharm Sci. 2012;4:505–7. [Google Scholar]
- 23.Gilhotra UK, Mohan G, Christina AJM. Antilithiatic activity of poly-herbal formulation tablets by in vitro method. J App Pharma Sci. 2013;3:43–8. [Google Scholar]
- 24.Hadjzadeh M, Rad AK, Rajaei Z, Mohammadian N, Valiollahi S, Sonei M. The beneficial effect of Cynodon dactylon fractions on ethylene glycol-induced kidney calculi in rats. Urol J Tehran. 2011;8:179–84. [PubMed] [Google Scholar]
- 25.Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, et al. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179–88. doi: 10.1016/j.phytochem.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep. 2005;57:70–6. [PubMed] [Google Scholar]
- 27.Fouada A, Yamina S, Nait MA, Mohammed B, Abdlekrim R. In vitro and in vivo antilithiasic effect of saponin rich fraction isolated from Herniaria hirsute. J Bras Nefrol. 2006;28:199–203. [Google Scholar]
- 28.Zhou J, Jin J, Li X, Zhao Z, Zhang L, Wang Q, et al. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-l-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis. 2017 doi: 10.1007/s00240-017-0985-y. doi:10.1007/s00240-017-0985-y. [DOI] [PubMed] [Google Scholar]
- 29.Atayoglu TA, Atayoglu GA, Gunduz O, Evren I, Silici S, Buchholz N. Propolis against urolithiasis. Fam Med Med Sci Res. 2015;4:152. [Google Scholar]
- 30.Wientarsih I, Madyastuti R, Prasetyo BF, Aldobrata A. Anti lithiasis activity of Avocado (Persea americana Mill) leaves extract in white male rats. Hayati J Biosci. 2012;19:49–52. [Google Scholar]
- 31.Prabhu VV, Sathyamurthy D, Ramasamy A, Das S, Anuradha M, Pachiappan S. Evaluation of protective effects of diosmin (a citrus flavonoid) in chemical-induced urolithiasis in experimental rats. Pharm Biol. 2016;54:1513–21. doi: 10.3109/13880209.2015.1107105. [DOI] [PubMed] [Google Scholar]
- 32.Grases F, Prieto RM, Fernandez-Cabot RA, Costa-Bauzá A, Tur F, Torres JJ. Effects of polyphenols from grape seeds on renal lithiasis. Oxidative Med Cellular Longevity. 2015. Available from: http://dx.doi.org/10.1155/2015/813737. [DOI] [PMC free article] [PubMed]
- 33.Saha S, Verma RJ. Polyphenolic extract of Piper nigrum inhibits sodium oxalate induced oxidative stress in rat kidney. Toxicol Environ Health Sci. 2014;6:164–9. [Google Scholar]
- 34.Yasir F, Wahab AT, Choudhary MI. Protective effect of dietary polyphenol caffeic acid on ethylene glycol-induced kidney stones in rats. Urolithiasis. 2017;1-10 doi: 10.1007/s00240-017-0982-1. doi:10.1007/s00240-017-0982-1. [DOI] [PubMed] [Google Scholar]
- 35.Torzewska A, Rozalski A. Inhibition of crystallization caused by Proteus mirabilis during the development of infectious urolithiasis by various phenolic substances. Microbiol Res. 2014;169:579–84. doi: 10.1016/j.micres.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Ghodasara J, Pawar A, Deshmukh C, Kuchekar B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacognosy Res. 2010;2:388–92. doi: 10.4103/0974-8490.75462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pande H, Kumar B, Varshney VK. Chapter-V. Results and discussions. [Last accessed on 2018 Jan 6]. Available from: http://shodhganga.inflibnet.ac.in/bitstream/10603/173683/15/15_chapter%205.pdf.
- 38.Hasan MR, Islam MN, Islam MR. Phytochemistry, pharmacological activities and traditional uses of Emblica officinalis: a review. Int Current Pharm J. 2016;5:14–22. [Google Scholar]
- 39.Bogucka-Kocka A, Zidorn C, Kasprzycka M, Szymczak G, Szewczyk K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J Biol Sci. 2016 doi: 10.1016/j.sjbs.2016.01.037. DOI: https://doi.org/10.1016/j.sjbs.2016.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


