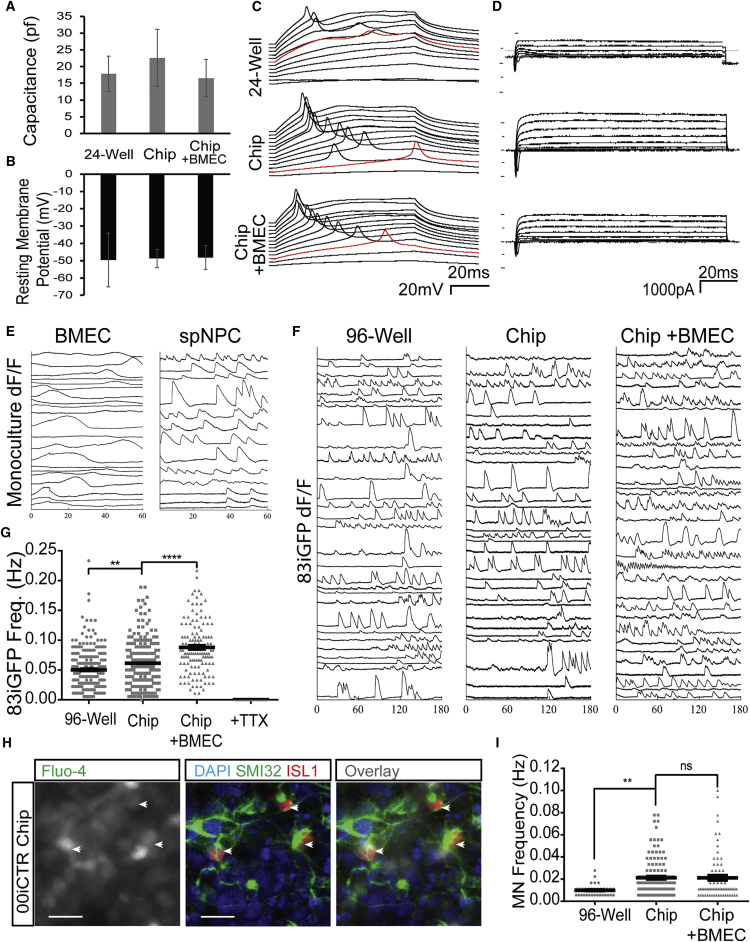
Figure 3.
Spinal Cord-Chip Environment Increases Spontaneous Neuronal Activity
(A and B) Average capacitance and resting membrane potential calculated at time of access to neuron during whole-cell current-clamp recording across three culture conditions: 24-well plate, Chip, and Chip containing BMECs (n = 5, 4, and 6 neurons, respectively).
(C) Membrane voltage recordings plotted over time during current-clamp. Traces are sequentially staggered for each 10-pA sweep. Red trace denotes minimum current sweep that reached action potential membrane threshold of 0 mV.
(D) Voltage-clamp recordings of membrane current over time. Picofarads (pf), milliseconds (ms), millivolts (mV), picoamperes (pA).
(E) Individual representative calcium transient activity of spNPC (top) and BMEC (bottom) cultures acquired at the seeding compartments where cultures do not interact. Change in florescence intensity over background (dF/F) plotted with respect to time totaling 60 s.
(F) Calcium transient activity plots of 30 representative neurons plotted over time in seconds and derived from 83iGFP spNPC cultures in each culture condition.
(G) Transient frequency plot of 128, 226, and 232 83iGFP-derived neurons cultured in 96-well plates, Chips, and Chips containing BMECs, respectively. Neuron activity was ablated with the administration of tetrodotoxin (TTX).
(H) Immunocytochemistry staining of islet1 (ISL1) and SMI32 (right) of site previously acquired for live calcium transients using Fluo-4 dye (left). ISL1-positive neurons (arrowheads) are superimposed to determine spMN firing specificity. Scale bars, 200 μm.
(I) Activity of ISL1 SMI32 double-positive neurons in three culture conditions plotted as frequency in hertz.
In graphs, means are represented by black bars and error bars represent standard error of the mean. Significance was calculated by one-way ANOVA: ∗∗p < 0.01, ∗∗∗∗p < 0.001; ns, not significant.