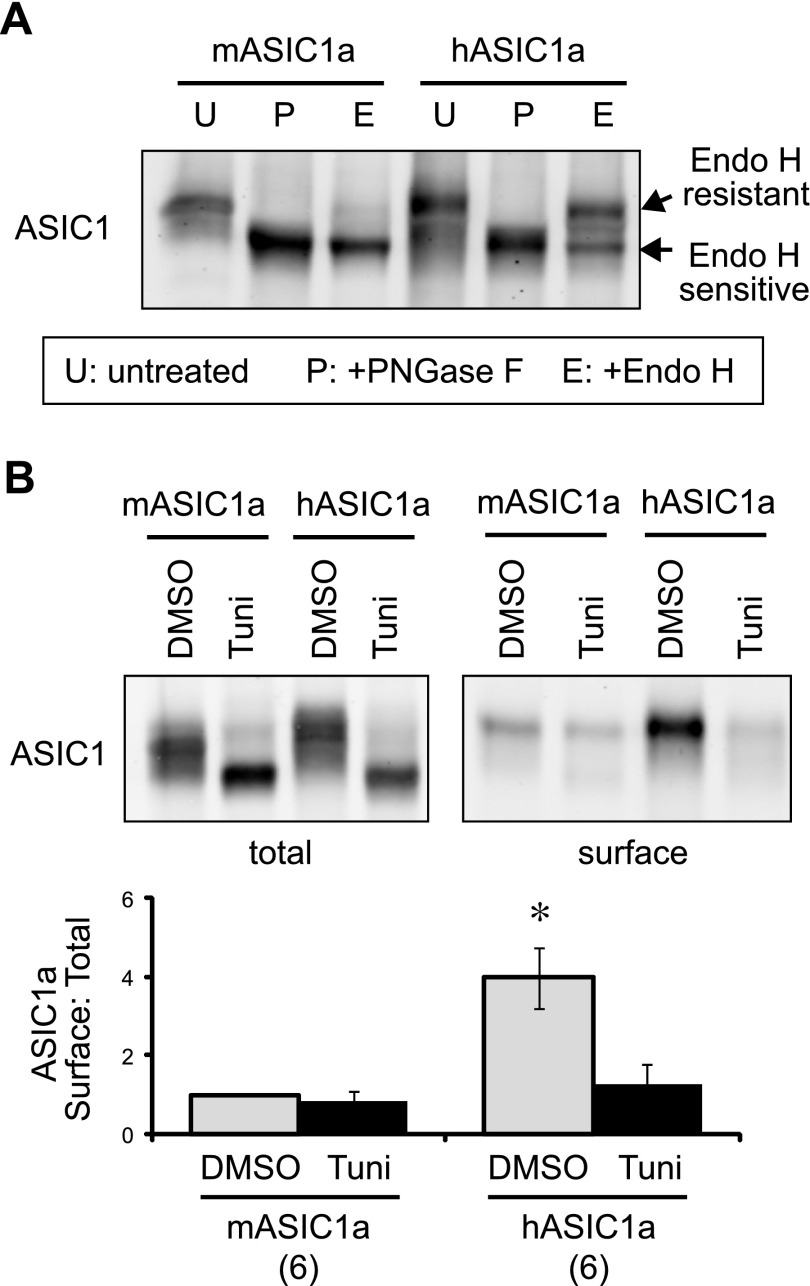
Figure 3.
Efficient N-glycosylation is responsible for higher surface trafficking of hASIC1a. A) CHO cells were transfected with hASIC1a or mASIC1a, lysed, and either untreated (U) or treated with PNGase F (P) or Endo H (E), as described in Materials and Methods. The migration patterns of ASIC1a were analyzed by Western blot. Note that PNGase F treatment removed all N-linked glycans, and the protein migrated faster on the gel. Endo H-treated samples migrated as 2 populations, and the slower-migrating population represents the population that carries matured (or processed) N-glycans, which are resistant to Endo H treatment. B) ASIC1a-expressing cells were treated with DMSO (vehicle control) or 0.5 µg/ml tunicamycin (Tuni) overnight. Surface biotinylation and analysis were performed as in Fig. 2B. Tunicamycin treatment reduced surface expression of hASIC1a and mASIC1a to a comparable level. Asterisk indicates that the vehicle-treated hASIC1a group is significantly different from all other groups. P < 0.01 (ANOVA).