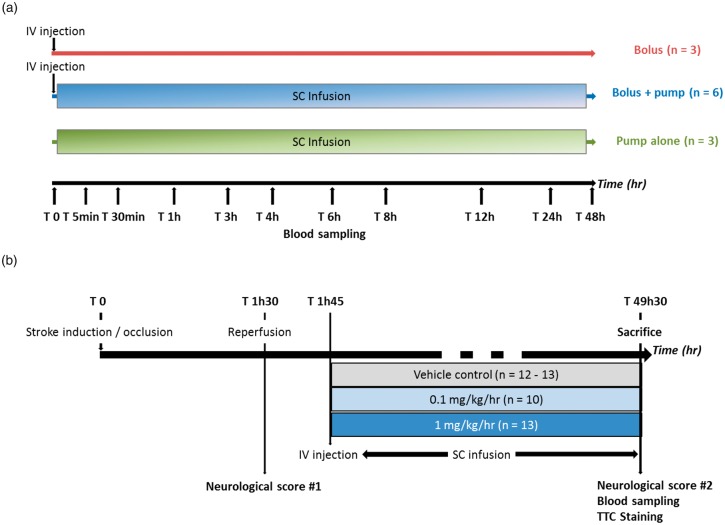
Figure 1.
Experimental design. (a) Experiment 1: Effect of delivery mode of (S)-roscovitine on pharmacokinetic parameters in healthy rats: Healthy rats received (S)-roscovitine at T0 either by IV bolus (25 mg/kg, n = 3), SC infusion (1 mg/kg/h for 48 h, n = 3) or both (25 mg/kg + 1 mg/kg/h for 48 h, n = 6). In order to measure (S)-roscovitine concentration in plasma, blood was collected at T0, T 5 min, T 30 min, T 1 h, T 3 h, T 6 h, T 8 h, T 24 h and T 48 h for bolus and bolus + pump groups and at T 0, T 1 h, T 4 h, T 12 h, T 24 h and T 48 h. (b) Experiment 2: Dose-response effect of (S)-roscovitine administration after transient middle cerebral artery occlusion on histopathological and neurological score in rats: Transient focal cerebral ischemia was induced in adult rats for 90 min. (S)-roscovitine or its vehicle control was administered 15 min post-reperfusion by an IV bolus (25 mg/kg body weight) followed by SC infusion for 48 hours: Vehicle (n = 12–13), 0.1 mg/kg/h (n = 10) or 1 mg/kg/h (n = 13). Neurological scoring was performed just prior to reperfusion and 48 h post-tMCAo. Blood was collected 48 h post-tMCAo to measure (S)-roscovitine concentration in plasma, then animals were euthanized and brain infarction volumes were measured by TTC staining.