Table 1.
In vitro affinities (Ki, μM) of α-truxillic acid and its congeners
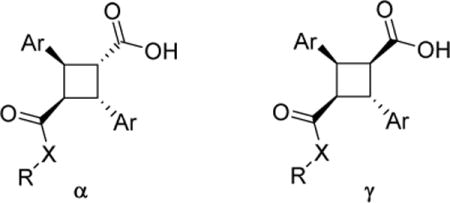
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Compound | Ar | X | R | FABP3 Ki | FABP5 Ki | FABP7 Ki | cLogP |
| 1 | 1a | Ph | O | H | >10 | >10 | >10 | 3.07 |
| 2 | 1g | 3,4-(MeO)2Ph | O | H | >10 | >10 | >10 | 2.38 |
| 3 | 1i | 3-OH-4-(MeO)Ph | O | H | >10 | >10 | >10 | 1.43 |
| 4 | 3 (racemic) | Ph | O | 1-naphthyl | 2.70 ± 0.42 | 0.81 ± 0.09 | 0.45 ± 0.07 | 6.97 |
| 5 | (R,R,R,R)-3 | Ph | O | 1-naphthyl | 3.26 ± 0.70 | 0.78 ± 0.14 | 0.89 ± 0.24 | 6.97 |
| 6 | (S,S,S,S)- 3 | Ph | O | 1-naphthyl | 2.82 ± 0.10 | 0.80 ± 0.14 | 0.66 ± 0.16 | 6.97 |
| 7 | 3a | Ph | O | benzyl | >10 | 3.81 ± 0.53 | 0.53 ± 0.12 | 5.28 |
| 8 | 3b | Ph | O | 4-MeO-benzyl | >10 | 2.15 ± 0.10 | 1.14 ± 0.06 | 5.20 |
| 9 | 3c | Ph | O | 4-F-benzyl | >10 | 2.42 ± 0.18 | 1.65 ± 0.21 | 5.42 |
| 10 | 3d | Ph | O | 4-Br-benzyl | >10 | 1.58 ± 0.16 | 1.25 ± 0.03 | 6.13 |
| 11 | 3e | Ph | O | 2-iodophenyl | 1.18 ± 0.10 | 1.34 ± 0.21 | 0.94 ± 0.34 | 5.77 |
| 12 | 3f | Ph | O | 3-ethynylphenyl | >10 | 0.89 ± 0.15 | 0.78 ± 0.12 | 5.06 |
| 13 | 3g | Ph | O | biphenyl-2-yl | 0.70 ± 0.42 | 0.77 ± 0.08 | 0.35 ± 0.12 | 6.12 |
| 14 | 3h | Ph | O | biphenyl-3-yl | 9.75 ± 0.79 | 0.85 ± 0.22 | 0.74 ± 0.17 | 6.68 |
| 15 | 3i | Ph | O | biphenyl-4-yl | 3.93 ± 0.16 | 2.52 ± 0.36 | 2.27 ± 0.03 | 6.68 |
| 16 | 3j | Ph | O | 2′-HO-biphenyl-2-yl | 3.52 ± 0.53 | 1.59 ± 0.43 | 0.54 ± 0.18 | 5.19 |
| 17 | 3k | Ph | O | 2,4,5-trichlorophenyl | 2.98 ± 0.85 | 0.80 ± 0.11 | 0.54 ± 0.02 | 6.67 |
| 18 | 3l | Ph | O | trans-2-phenylcyclohex-1-yl | 1.08 ± 0.37 | 0.21 ± 0.02 | 0.40 ± 0.03 | 7.17 |
| 19 | 3l-A | Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | 0.83 ± 0.15 | 0.21 ± 0.02 | 0.33 ± 0.05 | 7.17 |
| 20 | 3l-B | Ph | O | (1S,2R)-2-phenylcyclohex-1-yl | 0.88 ± 0.14 | 0.20 ± 0.03 | 0.25 ± 0.12 | 7.17 |
| 21 | 3l-A/C | Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | 0.64 ± 0.16 | 0.18 ± 0.03 | 0.33 ± 0.15 | 7.17 |
| 22 | 3l-B/D | Ph | O | (1S,2R)-2-phenylcyclohex-1-yl | 0.82 ± 0.09 | 0.21 ± 0.02 | 0.15 ± 0.02 | 7.17 |
| 23 | 3m | Ph | O | Indan-2-yl | >10 | 1.57 ± 0.15 | 2.41 ± 0.09 | 5.56 |
| 24 | 3n | Ph | O | CF3CH2- | >10 | >10 | 1.59 ± 0.24 | 3.87 |
| 25 | 3o | Ph | O | 6-acetamidonaphth-1-yl | 2.82 ± 0.18 | 0.97 ± 0.18 | 1.12 ± 0.45 | 5.10 |
| 26 | 3o-γ | Ph | O | 5-ethynylnaphth-1-yl | 3.56 ± 0.58 | 7.08 ± 0.44 | 7.43 ± 1.11 | 6.23 |
| 27 | 3p | Ph | O | 9-fluorenylmethyl | 4.94 ± 0.31 | 3.92 ± 0.75 | 1.03 ± 0.22 | 7.10 |
| 28 | 3q | Ph | O | cyclohexyl | >10 | 2.56 ± 0.16 | 2.70 ± 0.62 | 5.61 |
| 29 | 3r | Ph | O | 3-[1-(3,6,9-trioxadodecanyl)-1,2,3- triazol-4-yl]phenyl | >10 | 2.17 ± 0.32 | 0.50 ± 0.11 | 4.49 |
| 30 | 3s | Ph | O | 6-acetamidonaphth-1-yl | >10 | >10 | 1.06 ± 0.07 | 5.10 |
| 31 | 4a | 3-MeO-4-HO-Ph | O | 1-naphthyl | 1.06 ± 0.19 | >10 | 2.12 ± 0.19 | 4.33 |
| 32 | 4b | 2-MeO-Ph | O | 1-naphthyl | 0.69 ± 0.17 | 0.55 ± 0.05 | 0.67 ± 0.04 | 5.00 |
| 33 | 4c | 2-O2N-Ph | O | 1-naphthyl | >10 | >10 | >10 | 5.29 |
| 34 | 4d | 4-HO-Ph | O | 1-naphthyl | 2.30 ± 0.47 | >10 | 1.06 ± 0.34 | 4.63 |
| 35 | 4e | 2-MeO-Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | 0.40 ± 0.08 | 0.68 ± 0.06 | 0.40 ± 0.03 | 6.20 |
| 36 | 4f | 2-Cl-Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | >10 | 1.70 ± 0.33 | >10 | 8.59 |
| 37 | 4g | 2,6-Cl2−Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | >10 | 1.23 ± 0.18 | 6.32 ± 0.96 | 10.01 |
| 38 | 4h | 2-Br-Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | >10 | 2.76 ± 0.16 | >10 | 8.89 |
| 39 | 4i | 2-O2N-Ph | O | (1R,2S)-2-phenylcyclohex-1-yl | >10 | >10 | >10 | 6.49 |
| 40 | 4j | 2-MeO-Ph | O | 9-fluorenylmethyl | >10 | 1.72 ± 0.12 | >10 | 6.14 |
| 41 | 4k | 2-Cl-Ph | O | 9-fluorenylmethyl | >10 | 0.89 ± 0.05 | 3.54 ± 0.77 | 8.52 |
| 42 | 4l | 2-MeO-Ph | O | quinolin-5-yl | >10 | 3.93±0.51 | >10 | 4.89 |
| 43 | 5a | Ph | O | benzyl | >10 | >10 | >10 | 7.50 |
| 44 | 5b | Ph | O | 4-MeO-benzyl | >10 | >10 | >10 | 7.33 |
| 45 | 5c | Ph | O | 4-F-benzyl | >10 | >10 | >10 | 7.78 |
| 46 | 5d | Ph | O | tetrahydropyran-4-ylmethyl | >10 | >10 | >10 | 3.97 |
| 47 | 5e | Ph | O | biphenyl-3-yl | N.D.** | N.D.** | N.D.** | 10.29 |
| 48 | 6a | Ph | NH | 4-(5,6,7,8-tetrahydronaphth-2-yl)thiazol-2-yl | >10 | >10 | >10 | 7.48 |
| 49 | 6a-γ | Ph | NH | biphenyl-4-yl | >10 | >10 | >10 | 7.48 |
| 50 | 6b | Ph | NH | 4-(5,6,7,8-tetrahydronaphth-2-yl)thiazol-2-yl | >10 | >10 | >10 | 6.46 |
Ki values represent an average ± S.E. of at least three independent experiments. ** No data were obtained due to poor solubility.
