INTRODUCTION
Cancer is a major cause of morbidity and mortality in developing countries where health systems are poorly equipped to deal with this challenge.1 In the sub-Saharan region of Africa, the incidence of hematolymphoid malignancies is escalating in large part because of the human immunodeficiency virus (HIV) epidemic as well as population growth and aging.2 Despite the increasing burden, infrastructure for diagnosis and treatment of hematolymphoid malignancies remains inadequate in sub-Saharan Africa (SSA). In high-income countries (HICs), lymphoma diagnosis and classification rely heavily on expensive ancillary tools like flow cytometry, immunohistochemistry (IHC), and cytogenetics, which are, as yet, largely unavailable in SSA and other low-income to middle-income countries (LMICs). Likewise, intensive cytotoxic chemotherapy regimens are often not tolerable in settings with limited supportive care. Despite these limitations, strategies to improve care in SSA can be devised and successfully implemented. This article describes regional lymphoma epidemiology, current local approaches to laboratory and pathologic diagnosis, and ongoing research efforts in SSA.
REGIONAL LYMPHOMA EPIDEMIOLOGY
Although limited by data quality, GLOBOCAN 2012 estimates the age-standardized rate (ASR) of non-Hodgkin lymphoma (NHL) in SSA to be approximately 3.9 per 100,000 person years, with an age-standardized mortality of 3.2 per 100,000 person years.3 Classic Hodgkin lymphoma (CHL) further contributes to lymphoma burden with an ASR of 0.9%.3 Even when considering only expected growth and aging of the population without increasing incidence rates, the annual number of new lymphoma cases in SSA is expected to nearly double over the next 2 decades, from approximately 25,000 in 2012 to more than 48,000 by 2035.3
Lymphoma epidemiology in SSA is critically influenced by the important role of infectious disease in lymphomagenesis in this region. Oncogenic herpesviruses, HIV, and holoendemic malaria all contribute to a high burden of lymphoma and a disproportionate percentage of aggressive NHL.1,4
Among pediatric patients, endemic Burkitt lymphoma (eBL) predominates in much of SSA, representing more than 80% of all hematologic malignancies and more than 90% of NHLs in some published cohorts.5 eBL is almost invariably associated with Epstein-Barr virus (EBV) and follows a distribution that mirrors the malaria belt through central Africa.1,4 Although the precise mechanistic relationship between these pathogens remains incompletely understood, prevailing models suggest that malaria infection may either inhibit EBV-specific immune responses or promote EBV lytic reactivation, in either case ultimately leading to the development of eBL.6 Other common pediatric lymphoid malignancies in SSA include CHL, which is also generally EBV associated in this setting, and acute lymphoblastic leukemia/lymphoma (ALL).5,7,8 Although T-cell ALL (T-ALL) accounts for approximately 15% of pediatric ALL in Western cohorts, studies have shown an increased proportion of T-ALL compared with B-cell ALL in children and adolescents of African descent in the United States (25.8% vs 14%).9,10 Review of 58 cases of pediatric (<18 years old) ALL from Rwanda similarly reflects a higher proportion of T-ALL cases (36%) among Rwandan patients (Elizabeth Morgan, personal communication, 2017).
Among adults, lymphoma epidemiology is largely driven by the ongoing HIV epidemic in SSA. Since the early 1990s, lymphoma incidence has steadily increased throughout the region. For instance, in Kampala, Uganda, NHL ASR increased 3.0-fold and 2.3-fold in women and men, respectively, between 1991 and 2010.11 Although this trend is not entirely attributable to HIV, the epidemic is clearly the primary driver. HIV-associated lymphomas are disproportionately aggressive B-cell malignancies, such as diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), and plasmablastic lymphoma.7 These lymphomas are associated with high mortality in African cohorts, in which access to modern chemotherapy and supportive care is limited.12 Although improving access to antiretroviral therapy (ART) is likely to reduce the incidence of some HIV-associated malignancies, other lymphomas, including CHL and BL, are equally common or even more common in patients on ART.8 Hence, HIV is likely to be an important driver of lymphoma epidemiology in SSA for decades to come. Notably, in HICs with universal access to ART, lymphoma remains the primary cause of cancer-related death in individuals infected with HIV.13
Well-designed studies of lymphoma epidemiology in HIV-negative adults are generally lacking in SSA. Cancer registries tend to be limited by poor rates of histologic confirmation,14 and series with pathology-confirmed disease are likely to have considerable sampling bias, missing both patients with indolent lymphoma who choose not to present to medical care and patients with aggressive lymphomas who die at home before they can seek medical attention. Despite these limitations, the available data (including our own experience in Malawi) support a wide spectrum of B-cell and T-cell NHL and CHL.7 Indolent lymphomas and T-cell NHLs seem to represent a larger percentage of diagnoses in HIV-negative populations than in their HIV-positive counterparts.7
CURRENT THERAPIES AND INFRASTRUCTURE
Treatment of lymphoma at present in SSA is possible but also challenging.1 It should be emphasized that standard chemotherapy backbones for lymphoma treatment even in HIC typically use old drugs with generic formulations and can thus often be applied at acceptable cost even in LMICs, especially for curative-intent treatment. However, high HIV prevalence, high endemic opportunistic infection burden, and poor supportive care limit the complexity and intensity of the chemotherapy that can be safely administered in most SSA environments.1 Limited supportive care in particular includes supplies, personnel, and infrastructure for oncology nursing, infusional treatment, hematopoietic growth factor support, blood cultures, antimicrobial treatment, laboratory monitoring, and tumor lysis syndrome management (rasburicase, dialysis). Many groups are working to address these gaps and slowly build capacity, but there is clearly a cytotoxic ceiling for lymphoma treatment imposed by most SSA settings that is lower than for the same diseases in HIC. In addition, novel, targeted, noncytotoxic agents that have become accepted standards of care in HIC remain unavailable to most public sector patients in SSA, including rituximab, which received United States approval in 1991 and for which a less expensive, existing biosimilar is commercially available worldwide.15
As therapies become available and are shown to be effective and safe in SSA, patient selection through accurate diagnosis and classification is critical. For example, CD20 immunophenotyping is necessary for targeted therapy with rituximab, and expression by IHC is an inclusion criteria for safety and efficacy in an ongoing clinical trial in Malawi.16 Some patients screened for this study thought likely to have DLBCL had CD20-negative tumors. Without histopathologic and immunophenotypic confirmation, patients would have been misenrolled and mistreated, compromising their care and study objectives. Access to routine immunophenotyping can also serve as a tool for discovery, which is a common practice in HIC. Recently, human herpes virus 8 (HHV-8) multicentric Castleman disease was definitively identified in SSA by latency-associated nuclear antigen expression, leading to improved diagnosis and treatment.17,18
Adopting and implementing other advanced ancillary diagnostic testing is also within reach for LMIC. EBV plasma viral load can serve as an additional diagnostic, prognostic, and predictive biomarker for BL and additional applications in LMIC are being investigated.19 Thorough molecular classification and subcategorization of lymphoma cases from SSA have been completed in the United States.20 With appropriate infrastructure, such on-site testing can enhance and guide effective therapy.
Although piecemeal efforts to improve the treatment landscape are underway, a comprehensive regional solution is likely to be elusive until there are international, multilateral, public-private commitments for cancer similar to those that eventually made HIV treatment possible in SSA.21 Until then, many standard chemotherapy regimens from HIC likely can be safely and effectively applied in SSA at experienced centers with structured, resource-appropriate algorithms for monitoring and dose adjustment. These regimens include CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) for many aggressive NHL subtypes,12,22,23 CVP (cyclophosphamide, vincristine, prednisone) for indolent NHL, and ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) for CHL.24 For pediatric BL, regimens incorporating high-dose methotrexate, as implemented by the French-African Pediatric Oncology Group (GFAOP), have seemed more promising in SSA than anthracycline-based approaches,25–28 although nursing and supportive care requirements for safe systemic methotrexate administration are not trivial and require careful attention if this approach is to be successful. Regional standards for salvage chemotherapy after first-line failure, or for very aggressive, less common NHL subtypes like extranodal natural killer/T-cell lymphoma, are less clear. In addition, for lymphoma subtypes that are frequently causally associated with EBV, like CHL or pediatric BL, peripheral blood EBV measurement may be an implementable tool in SSA to allow better prognostication and response assessment during treatment, potentially optimizing the therapeutic index and minimizing treatment-related morbidity, in light of the supportive care limitations highlighted earlier.19,24
INADEQUACY OF PATHOLOGY SERVICES
Tissue diagnosis is essential in the provision of clinical and public health services for cancer.29 From screening for malignant disease; through diagnosis, staging, guiding, and monitoring treatment; to evaluating the complications of treatment, every step requires the support of pathology investigations.30 In a review of a population-based cancer registry data in Malawi, just 18% of all cancer diagnoses had a pathologically confirmed diagnosis. The remaining cancers were diagnosed based on clinical suspicion alone or with the aid of radiology in some cases.14 As therapies become more accessible, accurate tissue-based diagnosis will prevent overtreatment/undertreatment of potentially curable diseases.
Adequate pathology services are particularly important in the accurate diagnosis and classification of lymphomas, which may clinically mimic infectious diseases like tuberculosis. Striking differences still exist in the reliability of lymphoma diagnosis between HIC and LMIC. In 2011, the International Network of Cancer Treatment and Research (INCTR) conducted an evaluation of infrastructure for diagnosing lymphoma in 4 sub-Saharan countries: Kenya, Tanzania, Nigeria, and Uganda.31 Key findings included wide use of fine-needle aspiration cytology as a diagnostic tool, lack of IHC or other immunophenotyping tools, variable equipment and personnel, and variable turnaround times. Many of the histologic and cytologic preparations were deemed suboptimal. However, as expected, metrics of quality were highly tied to available resources, and some centers collaborating with institutions in developed countries had well-established infrastructure and turnaround times of less than a week. Overall, pathologists were well trained but few in number, with inadequate numbers of trainees. Molecular and cytogenetic techniques, which are core elements of hematopathology, were not available in any of the centers.31
In light of the significant need, collaborative efforts to improve diagnostic pathology services in the region are ongoing at many levels. For instance, the National Cancer Institute (NCI) is currently supporting the Sub-Saharan Africa Lymphoma Consortium, a grouping of 10 regional referral centers with an aim of characterizing lymphomas. In large part because of international investments such as this one, substantial progress has been made in recent years.
KAMUZU CENTRAL HOSPITAL, MALAWI
Although diagnostic pathology services remain insufficient in the region, they are not entirely absent.32 General uniformity and structure between programs is lacking, and infrastructure, staffing, and financial support are highly variable. In some areas, support from government and/or partnering academic institutions is significant, and laboratory capacity matches that in HIC. In contrast, some laboratories depend heavily on volunteers and donations, which adds logistical complexity.
The hematopathology services at the Kamuzu Central Hospital (KCH) were developed more recently, through the close collaborative efforts of the Malawi Ministry of Health and the University of North Carolina (UNC). Located in the capital city of Lilongwe, KCH is one of 2 national teaching hospitals in Malawi and has a referral base of approximately 4 million to 5 million people. The laboratory was established in 2011 with financial backing from both institutional and governmental sources.
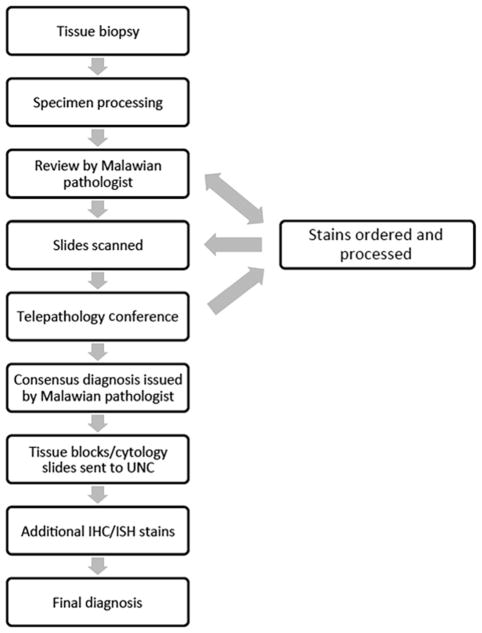
In 2016, the laboratory processed a total of 5611 histology and 1998 cytology cases. Most of these cases were interpreted by one of 2 local pathologists without international consultation or additional stains. However, difficult cases, as well as diagnostic specimens from all patients enrolled in the ongoing KCH Lymphoma Study, are additionally reviewed at a weekly telepathology conference attended by Malawian clinicians, Malawian pathologists, and their counterparts in the United States. The basic work flow for such cases is outlined in Fig. 1. Briefly, bone marrow and tissue biopsies or cytology specimens are processed and then initially reviewed by a local pathologist, who develops an impression, which is often shared with the treating clinician. In some cases, an initial panel of IHC stains may be ordered (Box 1). Relevant slides are scanned using an Aperio slide scanner and then uploaded to a secure online server. Cases are then reviewed in a weekly telepathology conference attended by Malawi-based clinicians and pathologists, as well as their colleagues in the United States. After discussion at this conference, and often after additional rounds of IHC stains, a consensus diagnosis is rendered by the local pathologist.
Fig. 1.
KCH pathology laboratory work flow overview. ISH, in situ hybridization.
Box 1. Immunohistochemical stains available at the Kamuzu Central Hospital pathology laboratory.
| Available stains: |
| CD45 |
| CD3 |
| CD20 |
| Ki67 |
| TdT |
| BCL2 |
| CD30 |
| CD138 |
| HHV8 (latency-associated nuclear antigen) |
| Synaptophysin |
| AE1/AE3 |
To ensure quality control and to facilitate research studies, tissue blocks and glass cytology slides are sent to collaborators in the United States quarterly. Histology cases are then further characterized by a broader panel of IHC and in situ hybridization stains. After glass slide review and expanded immunophenotyping, a final diagnosis is rendered. In a cohort of KCH patients with lymphoma and other lymphoproliferative disorders, concordance between initial diagnosis in Malawi and final diagnosis in the United States was very high.8 Although expanded immunophenotyping permits more granular classification of some lymphomas, the initial diagnosis made in Malawi led to treatment appropriate to this setting in 95% of cases.
In addition to the immediate impacts on patient care, the robust pathology service at KCH has had several additional impacts. First, as described later, it has facilitated involvement in several regional and international clinical trials. Second, and equally important, the growth of services and infrastructure has attracted Malawian pathologists and technologists to a center that lacked any pathology services just 6 years ago.
ONGOING REGIONAL AND INTERNATIONAL CLINICAL TRIALS
In addition to important individual efforts, there are 2 cooperative groups sponsored by the NCI focused on lymphoma treatment in SSA. First, the AIDS Malignancy Consortium includes several SSA centers, and to date has implemented treatment studies for Kaposi sarcoma and cervical cancer. A clinical trial is also being implemented for HIV-associated DLBCL, with patients randomized to receive either CHOP or low-dose oral metronomic treatment, based on results from an earlier pilot study in Kenya and Uganda for this population before ART was widely available.33,34 NCI is also working to convene a pediatric BL network spanning multiple SSA countries. Similar multicenter studies for pediatric BL specifically have been successfully completed by GFAOP and INCTR, as well as other groups.25,35,36 All of these efforts convincingly suggest that harmonized studies across countries using a standardized protocol is achievable in SSA even for complex interventions like lymphoma treatment, and continued support for these efforts is vital to test innovative approaches and define optimal standards of care in LMIC.
FUTURE DIRECTIONS
Diagnostic pathology services are paramount for routine care and implementing impactful lymphoma trials. Although substantive progress has been made to document the extent to which pathology services are lacking, resources necessary to help guide the development and/or sustainability of anatomic pathology laboratories in the region are limited. Creation of universal best-practice guidelines, however, may be unrealistic because of intraregional variability in existing laboratory infrastructure, support, and staffing. Nonetheless, defining local needs, developing sustainable fiscal models to support pathology, and implementing quality-assurance standards are necessary for wider scale-up of lymphoma diagnostics in SSA.
Intensive laboratory accreditation processes necessary to maintain diagnostic consistency and accuracy in HICs. Although these standards are incrementally being addressed in the clinical pathology laboratories in SSA,37–40 the anatomic pathology services required for lymphoma diagnosis lag behind. This difference likely reflects complexities of more manual (rather than automated) testing and paucity of infrastructure and expertise. However, as obstacles to lymphoma treatment in SSA are overcome, there is increasing need for ongoing, region-specific proficiency testing and laboratory accreditation processes. Comprehensive assessment of current pathology laboratory capacity is clearly necessary, as are creative approaches to testing and sustainability. Collaborative approaches and resource sharing may significantly improve diagnostic quality throughout the region.
SUMMARY
The care of patients with lymphoma relies heavily on accurate tissue diagnosis. However, in SSA, where lymphoma burden is increasing because of population growth, aging, and persistent epidemic levels of HIV infection, diagnostic pathology services are often inadequate to support resource-appropriate lymphoma care. Improvement of regional capacity to accurately classify tumors is paramount to understanding true disease epidemiology and to establish effective treatment strategies. Although regionally lacking, successful pathology laboratories capable of supporting strong lymphoma clinical and research efforts have been established. Continued development of sustainable diagnostic approaches and implementation of quality control initiatives are likely to have far-reaching effects on improving lymphoma patient outcomes in the region.
KEY POINTS.
Clinical and pathologic studies of patients not infected with human immunodeficiency virus with hematolymphoid malignancies are severely lacking in sub-Saharan Africa (SSA), but are necessary to inform and support effective treatment strategies.
Most chemotherapeutic agents used for lymphoma treatment are inexpensive and available. As such, access to these therapies depends on accurate diagnosis and not necessarily funding.
Targeted therapies for some lymphomas require specific diagnostic tools beyond the standard histology that is implementable in SSA.
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose.
References
- 1.Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119(22):5078–87. doi: 10.1182/blood-2012-02-387092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One. 2013;8(8):e70361. doi: 10.1371/journal.pone.0070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [Accessed December 14, 2015]. Available at: http://globocan.iarc.fr. [Google Scholar]
- 4.Rogena EA, De Falco G, Schurfeld K, et al. A review of the trends of lymphomas in the equatorial belt of Africa. Hematol Oncol. 2011;29(3):111–5. doi: 10.1002/hon.977. [DOI] [PubMed] [Google Scholar]
- 5.Sinfield RL, Molyneux EM, Banda K, et al. Spectrum and presentation of pediatric malignancies in the HIV era: experience from Blantyre, Malawi, 1998–2003. Pediatr Blood Cancer. 2007;48(5):515–20. doi: 10.1002/pbc.20917. [DOI] [PubMed] [Google Scholar]
- 6.Moormann AM, Bailey JA. Malaria - how this parasitic infection aids and abets EBV-associated Burkitt lymphomagenesis. Curr Opin Virol. 2016;20:78–84. doi: 10.1016/j.coviro.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery ND, Liomba NG, Kampani C, et al. Accurate real-time diagnosis of lymphoproliferative disorders in Malawi through clinicopathologic teleconferences: a model for pathology services in sub-Saharan Africa. Am J Clin Pathol. 2016;146(4):423–30. doi: 10.1093/ajcp/aqw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20(12):1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350(15):1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, Sandlund JT, Pei D, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290(15):2001–7. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 11.Wabinga HR, Nambooze S, Amulen PM, et al. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer. 2014;135(2):432–9. doi: 10.1002/ijc.28661. [DOI] [PubMed] [Google Scholar]
- 12.Gopal S, Fedoriw Y, Kaimila B, et al. CHOP chemotherapy for aggressive non-Hodgkin lymphoma with and without HIV in the antiretroviral therapy era in Malawi. PLoS One. 2016;11(3):e0150445. doi: 10.1371/journal.pone.0150445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–18. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gota V, Karanam A, Rath S, et al. Population pharmacokinetics of Reditux, a bio-similar rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78(2):353–9. doi: 10.1007/s00280-016-3083-x. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed August 22, 2017];Rituximab plus CHOP chemotherapy for diffuse large B-cell lymphoma. Available at: https://clinicaltrials.gov/ct2/show/NCT02660710.
- 17.Gopal S, Liomba NG, Montgomery ND, et al. Characteristics and survival for HIV-associated multicentric Castleman disease in Malawi. J Int AIDS Soc. 2015;18:20122. doi: 10.7448/IAS.18.1.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopal S, Fedoriw Y, Montgomery ND, et al. Multicentric Castleman’s disease in Malawi. Lancet. 2014;384(9948):1158. doi: 10.1016/S0140-6736(14)61366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westmoreland KD, Montgomery ND, Stanley CC, et al. Plasma Epstein-Barr virus DNA for pediatric Burkitt lymphoma diagnosis, prognosis and response assessment in Malawi. Int J Cancer. 2017;140(11):2509–16. doi: 10.1002/ijc.30682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan E, Sweeny MP, Tomoka T, et al. Targetable subsets of non-Hodgkin lymphoma in Malawi define therapeutic opportunities. Blood Adv. 2016;1(1):84–92. doi: 10.1182/bloodadvances.2016000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopal S. Moonshot to Malawi. N Engl J Med. 2016;374(17):1604–5. doi: 10.1056/NEJMp1601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Witt P, Maartens DJ, Uldrick TS, et al. Treatment outcomes in AIDS-related diffuse large B-cell lymphoma in the setting roll out of combination antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2013;64(1):66–73. doi: 10.1097/QAI.0b013e3182a03e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bateganya MH, Stanaway J, Brentlinger PE, et al. Predictors of survival after a diagnosis of non-Hodgkin lymphoma in a resource-limited setting: a retrospective study on the impact of HIV infection and its treatment. J Acquir Immune Defic Syndr. 2011;56(4):312–9. doi: 10.1097/QAI.0b013e31820c011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westmoreland KD, Stanley CC, Montgomery ND, et al. Hodgkin lymphoma, HIV, and Epstein-Barr virus in Malawi: longitudinal results from the Kamuzu Central Hospital lymphoma study. Pediatr Blood Cancer. 2017;64(5) doi: 10.1002/pbc.26302. https://doi.org/10.1002/pbc.26302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa–report of the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2008;50(6):1138–42. doi: 10.1002/pbc.21452. [DOI] [PubMed] [Google Scholar]
- 26.Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173(5):705–12. doi: 10.1111/bjh.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molyneux E, Schwalbe E, Chagaluka G, et al. The use of anthracyclines in the treatment of endemic Burkitt lymphoma. Br J Haematol. 2016 doi: 10.1111/bjh.14440. https://doi.org/10.1111/bjh.14440. [DOI] [PubMed]
- 28.Buckle G, Maranda L, Skiles J, et al. Factors influencing survival among Kenyan children diagnosed with endemic Burkitt lymphoma between 2003 and 2011: a historical cohort study. Int J Cancer. 2016;139(6):1231–40. doi: 10.1002/ijc.30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e152–7. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 30.Stefan DC. Childhood cancer in Africa: an overview of resources. J Pediatr Hematol Oncol. 2015;37(2):104–8. doi: 10.1097/MPH.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 31.Naresh KN, Raphael M, Ayers L, et al. Lymphomas in sub-Saharan Africa–what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? Br J Haematol. 2011;154(6):696–703. doi: 10.1111/j.1365-2141.2011.08772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mpunga T, Tapela N, Hedt-Gauthier BL, et al. Diagnosis of cancer in rural Rwanda: early outcomes of a phased approach to implement anatomic pathology services in resource-limited settings. Am J Clin Pathol. 2014;142(4):541–5. doi: 10.1309/AJCPYPDES6Z8ELEY. [DOI] [PubMed] [Google Scholar]
- 33.Mwanda WO, Orem J, Fu P, et al. Dose-modified oral chemotherapy in the treatment of AIDS-related non-Hodgkin’s lymphoma in East Africa. J Clin Oncol. 2009;27(21):3480–8. doi: 10.1200/JCO.2008.18.7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. [Accessed August 22, 2017];Intravenous chemotherapy or oral chemotherapy in treating patients with previously untreated stage III-IV HIV-associated non-Hodgkin lymphoma. Available at: https://clinicaltrials.gov/ct2/show/NCT01775475.
- 35.Ngoma T, Adde M, Durosinmi M, et al. Treatment of Burkitt lymphoma in equatorial Africa using a simple three-drug combination followed by a salvage regimen for patients with persistent or recurrent disease. Br J Haematol. 2012;158(6):749–62. doi: 10.1111/j.1365-2141.2012.09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hesseling PB, Molyneux E, Tchintseme F, et al. Treating Burkitt’s lymphoma in Malawi, Cameroon, and Ghana. Lancet Oncol. 2008;9(6):512–3. doi: 10.1016/S1470-2045(08)70139-6. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder LF, Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141(6):791–5. doi: 10.1309/AJCPQ5KTKAGSSCFN. [DOI] [PubMed] [Google Scholar]
- 38.Kibet E, Moloo Z, Ojwang PJ, et al. Measurement of improvement achieved by participation in international laboratory accreditation in sub-Saharan Africa: the Aga Khan University Hospital Nairobi experience. Am J Clin Pathol. 2014;141(2):188–95. doi: 10.1309/AJCPV8A9MRWHGXEF. [DOI] [PubMed] [Google Scholar]
- 39.Amukele TK, Michael K, Hanes M, et al. External quality assurance performance of clinical research laboratories in sub-Saharan Africa. Am J Clin Pathol. 2012;138(5):720–3. doi: 10.1309/AJCP8PCM4JVLEEQR. [DOI] [PubMed] [Google Scholar]
- 40.Gershy-Damet GM, Rotz P, Cross D, et al. The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol. 2010;134(3):393–400. doi: 10.1309/AJCPTUUC2V1WJQBM. [DOI] [PubMed] [Google Scholar]