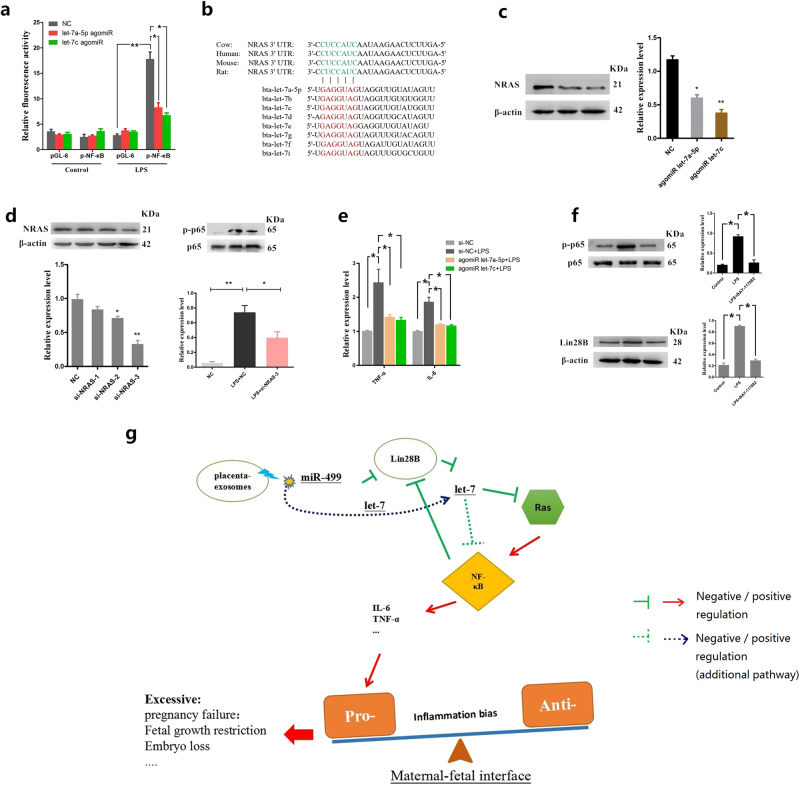
Fig. 7. Bta-let-7 miRNAs inhibit the activation of NF-κB signaling and expression of proinflammatory cytokines.
a BEND cells were transfected with either mixed let-7a-5p/let-7c agomiR or a control miR-NC (agomiR-NC), along with the control pGL6 vector or the pNF-κB luciferase reporter. At 24 h after transfection, the cells were treated with 1 μg/mL LPS for 1 h or left untreated as a control. Luciferase activities were determined by a dual-luciferase assay system. b Conservation of the let-7 target sequence in NRAS among different species (upper panel), and a common seed sequence of bta-let-7 miRNA families (lower panel). c BEND cells were transfected with bta-let-7a-5p and bta-let-7c agomiRs or the control oligonucleotide, and NRAS protein levels were then determined by western blotting. β-actin was used as a control. d BEND cells were transfected with NRAS siRNA, and the expression levels of NRAS or phosphorylation levels of NF-κB p65 were detected by western blotting. β-actin was used as a control. e BEND cells were transfected with bta-let-7a-5p and bta-let-7c agomiRs or the control oligonucleotide (NC), and the mRNA levels of the proinflammatory cytokines TNF-α and IL-6 were quantified by qPCR; GAPDH was used as the internal control. f BAY-117082 (20 μM) was used to block NF-κB, and then Lin28B protein levels were determined by western blotting. β-actin was used as a control. Data represent three independent experiments and are presented as the mean ± S.E.M. (error bars). Two-tailed Student’s t-test, *P < 0.05; **P < 0.01. g Schematic diagram of placental exosomes in the regulation of the uterine immune inflammatory response. During early pregnancy, the proinflammatory uterine immune microenvironment leads to the activation of NF-κB and promotes the transcriptional expression of downstream proinflammatory cytokines, such as TNF-α and IL-6. However, to maintain an appropriate, proinflammatory environment, placental exosomes target the Lin28B/let-7-ras signaling axis through miR-499 to directly or indirectly inhibit NF-κB activation and attenuate transcriptional regulation of downstream proinflammatory cytokines, resulting in a mild proinflammatory environment. However, activation of NF-κB also promotes Lin28B expression, which counters the continued inhibition of miR-499. Moreover, an additional complementary pathway through which placental exosome-derived let-7 miRNAs are involved in this process was identified. Collectively, placental exosome-derived bta-miR-499, Lin28B, and bta-let-7 miRNAs constitute a loop that negatively regulates NF-κB p65 activation, contributing to the pro/anti-inflammatory balance at the maternal-fetal interface during early pregnancy in cattle