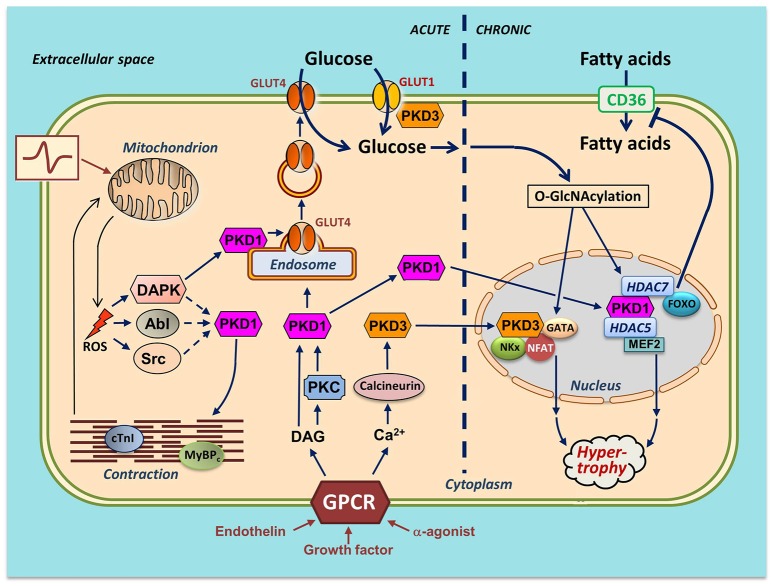
Figure 2.
Integration of actions of PKD1 and PKD3 in cardiac energy metabolism—a hypothetical scheme. Several stimuli induce activation of PKDs in cardiomyocytes. Endothelin, growth factors and α-adrenergic agonists activate distinct members of the family of G protein-coupled receptors (GPCR), leading to sarcolemmal phospholipase C activation and subsequent increases in diacylglycerol (DAG) and Ca2+. DAG, as part of the canonical PKD1 activation pathway, binds to PKD1 thereby bringing it to the sarcolemma for further activation by PKCs. From there, the activated PKD1 migrates either to the nucleus or to endosomes. Please note that the sarcolemmal activation step of the cytoplasmic PKD1 pool is not displayed. In the nucleus, PKD1 phosphorylates the histone deacetylases (HDAC)5 and HDAC7. HDAC5 phosphorylation results in release and activation of the hypertrophic transcription factor MEF2. HDAC7 phosphorylation leads to binding to and inhibition of FOXO1, thereby depressing CD36 expression and cellular fatty acid uptake. In the cytosol, DAG stimulates PKD1 to migrate to the endosomes to acutely stimulate GLUT4 translocation and glucose uptake. Increased Ca2+ stimulates calcineurin-induced upregulation of PKD3 and activation of three additional hypertrophic transcription factors (GATA, NFAT, and NKx). PKD3 also regulates sarcolemmal GLUT1 localization. An increase in physical work (contractile activity) increases mitochondrial formation of ROS as by-product of elevated oxidative phosphorylation flux. DAPK migrates to the endosomes to activate the endosomally resident PKD1 pool to initiate vesicle-mediated GLUT4 translocation. DAPK, as well as Abl/Src may stimulate phosphorylation of myofilament proteins thereby further stimulating contractile activity. mROS production activates the Ser/Thr-kinase DAPK and the Tyr-kinases Abl and Src. For clarity, the effects of the PKD are divided into acute (signaling, metabolism and contraction; left part of figure) and chronic (nuclear; right part of figure). It is of importance to note that PKD1 and PKD3 integrate cardiac metabolism with contraction and also with remodeling. In case of integration of metabolism with contraction (as occurs upon an acute increase in workload), PKD1 activation ensures that the increase in contractility occurs hand in hand with increased glucose uptake. If the workload transcends from acute into chronic (as occurs upon chronic pressure overload), the PKDs are also needed for integration of metabolism with remodeling. Specifically, chronically elevated GLUT4 translocation and elevated GLUT1 expression together lead to increased glucose uptake and subsequent O-GlcNAcylation of myocellular proteins including the hypertrophic transcription factors. Together, the PKD1/PKD3-mediated direct activation of the transcription factors and the PKD1/PKD3 mediated O-GlcNAcylation and further activation of the transcription factors may lead to full-blown hypertrophic cardiac remodeling.