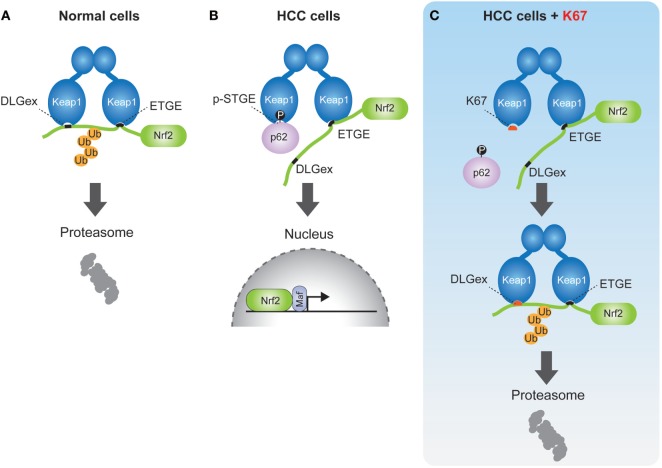
Figure 3.
Schematic model of nuclear factor erythroid 2-related factor 2 (Nrf2) activation by phospho-p62 (A). In normal cells, the ETGE and DLGex motifs of Nrf2 bind to the Keap1DC domain, which promotes ubiquitination of Nrf2 followed by its proteasomal degradation. (B) In human hepatocellular carcinoma cells, phospho-p62 (pS349) competitively interacts with the DLGex site on Keap1DC, resulting in the translocation of Nrf2 into nucleus. (C) K67 binds to Keap1DC at higher affinity than phosphor-p62, whereas the DLGex motif of Nrf2 binds to Keap1DC with covering K67. Nrf2 is eventually ubiquitinated and degraded by the proteasome.