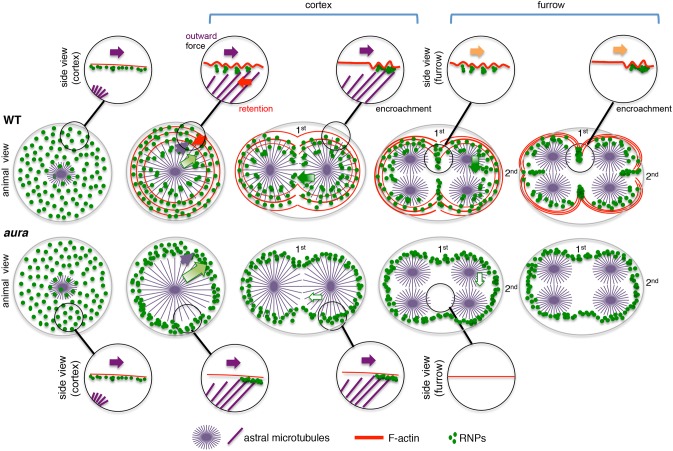
Fig. 10.
Model for the Mid1ip1L- and F-actin-dependent modulation of germ plasm segregation. In wild type (WT, animal view) during the first few cell cycles, Mid1ip1L-dependent F-actin dynamics attenuate (red arrow) a microtubule-dependent outward movement (purple arrows), resulting in the regulated outward movement of RNPs (graded light-green arrows). Regulated outward RNP movement allows the partial persistence of RNPs at the cortex, making them available for recruitment to the furrows for the next cell cycles (graded dark-green arrows). In aura mutants (animal view), Mid1ip1L-dependent modulation is affected, causing unregulated outward RNP movement. This results in low persistence of RNPs at the cortex, leading to reduced RNP furrow recruitment (white arrows) and increased peripheral accumulation. Insets as side views depict shared aspects of Mid1ip1L-dependent surface contractions at the cortex and along the furrow, the gradual increase in density of which facilitates RNP aggregate fusion through encroachment. Growing astral microtubules likely provide a directional outward radial cue to influence cortical contractions. A second signal (yellow arrows) provides medial-to-distal directionality along the furrow [see accompanying work (Eno et al., 2018); furrow-associated microtubules are not shown].