Table 1. Optimization of triethylborane-promoted tricyclopentane ring-opening.
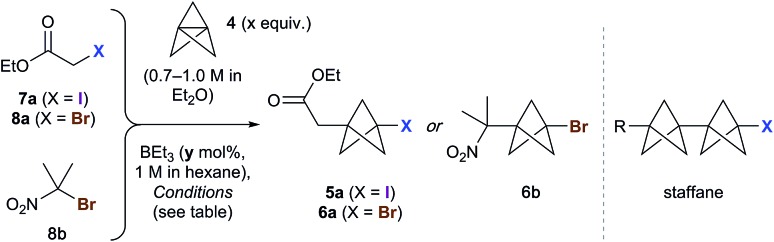
| ||||||
| Entry | Substrate | x (equiv.) | y (mol%) | T | t | Yield a (%) |
| 1 | 7a | 1.3 | 10 | rt | 15 min | 83 |
| 2 | 7a | 1.3 | 10 | 0 °C | 15 min | 87 |
| 3 | 7a | 1.3 | 5 | 0 °C | 15 min | 89 |
| 4 | 7a | 1.3 | 1 | 0 °C | 15 min | 95 |
| 5 | 7a | 1.3 | 0.5 | 0 °C | 15 min | (67 : 33) |
| 6 | 7a | 1.1 | 1 | 0 °C | 15 min | 92 |
| 7 | 7a | 2.0 | 10 | rt | 16 h | 98 b |
| 8 | 7a | 2.0 | 0 | rt | 20 h | (60 : 40) c |
| 9 | 7a | 2.0 | 0 | rt | 20 h | (33 : 67) |
| 10 | 8a | 1.3 | 10 | rt | 20 h | 47 d |
| 11 | 8b | 1.3 | 10 | rt | 15 min | 67 |
| 12 | 8b | 1.3 | 10 | 0 °C | 15 min | 73 |
| 13 | 8b | 1.3 | 1 | 0 °C | 15 min | (60 : 40) |
aIsolated yields. Figures in parentheses indicate incomplete reactions, and the ratio of starting material to product as judged by 1H NMR spectroscopic analysis of the crude reaction mixture.
bReaction carried out in Bu2O (0.19 M).
cReaction carried out in the dark.
dIsolated as a 6 : 1 mixture of 6a : staffane.
