Figure 2.
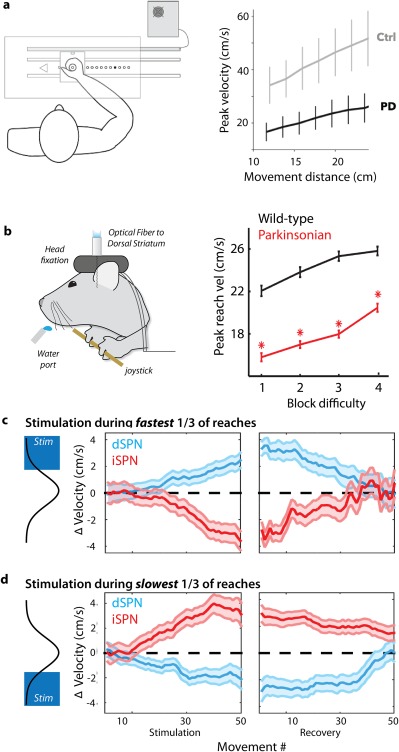
Regulation of movement vigor by opponent BG pathways. (a) Dopamine depletion from Parkinson's disease in patients (unmedicated patients with chronic DBS stimulators inactivate during the experiment) produces a robust slowing of movement across a broad range of movement vigor relative to control subjects (Ctrl). Modified from reference 9. Parkinsonian patients and controls were instructed to move a joystick to several distances indicated by an LED illuminated at the target eccentricity (example of a human VAO task). (b) Mice were required to perform a similar VAO task, moving a joystick to increasing amplitudes. Dopamine depletion in the MitoPark103 murine model of PD (“parkinsonian”) spares the ability to select and initiate the proper action, but reduces the speed of movement across a range of movement vigor. (c, d) Selective, closed‐loop optogenetic stimulation (pulsed for 450 milliseconds, < 10 milliseconds after movement onset) of direct pathway dSPNs (blue) or indirect pathway iSPNs (red) during the fastest‐reaching movements induces a cumulative shift in peak reach speed compared with control sessions (zero). Changes were a form of learning that persisted for tens of trials of the no‐stimulation recovery period. This effect extends to all reaches, not only those reaches during which stimulation occurred (as evidenced by a lack of change in in the width of the SEM bars throughout the session). The converse was observed when stimulation occurred during the slowest movements — direct pathway stimulation induced a slowing of movement, whereas indirect pathway stimulation induced a speeding of movement. Modified from reference 56.
