Figure 3.
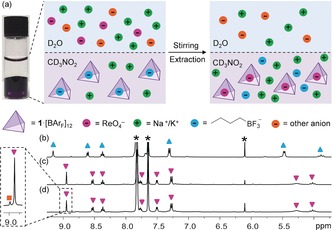
a) Selective liquid–liquid extraction of ReO4 − in the presence of other anions. Conditions: 0.8 mm nBuBF3 −⊂1⋅[BArF]11 in CD3NO2; 0.8 mm in D2O of each of NaReO4, NaF, NaCl, NaBr, NaI, Na2SO4, KClO4, KNO3, NaBF4, NaH2PO4, and NaOAc; 7 hours stirring at RT; b–d) Partial 1H NMR spectra of (b) the CD3NO2 phase before extraction, showing only the presence of nBuBF3 −⊂1⋅[BArF]11 (blue ▴); c) the CD3NO2 phase after extraction in the absence of competing anions, showing only the presence of ReO4 −⊂1⋅[BArF]11 (pink ▾); (d) the CD3NO2 phase after extraction in the presence of competing anions, showing the presence of 97 % ReO4 −⊂1⋅[BArF]11 (pink ▾) and 3 % ClO4 −⊂1⋅[BArF]11 (orange ▪). The peaks of BArF − and the trimethoxybenzene standard are denoted by asterisks.
