Figure 1.
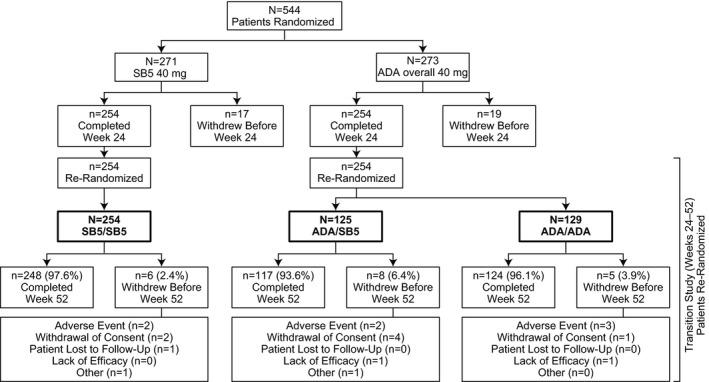
Patient disposition summary. The percentages of patients who completed or discontinued are based on the numbers of patients who were rerandomized at week 24. The SB5 group included patients who were randomized to receive the biosimilar SB5 at week 0. The adalimumab (ADA) overall group included patients who were randomized to receive reference ADA at week 0. The SB5/SB5 group included patients receiving SB5 who were rerandomized to continue SB5. The ADA/SB5 group included patients receiving ADA who were rerandomized to switch to SB5. The ADA/ADA group included patients receiving ADA who were rerandomized to continue ADA.
