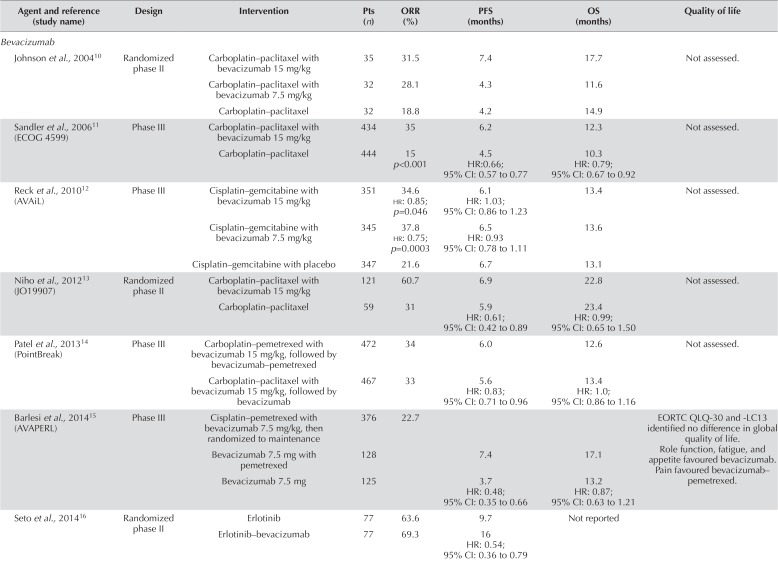
TABLE I.
Summary of trials evaluating intravenous antiangiogenic therapies in first-line systemic therapy for non-small-cell lung cancer
| Agent and reference (study name) | Design | Intervention | Pts (n) | ORR (%) | PFS (months) | OS (months) | Quality of life |
|---|---|---|---|---|---|---|---|
| Bevacizumab | |||||||
| Johnson et al., 200410 | Randomized phase II |
Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 35 | 31.5 | 7.4 | 17.7 | Not assessed. |
| Carboplatin–paclitaxel with bevacizumab 7.5 mg/kg | 32 | 28.1 | 4.3 | 11.6 | |||
| Carboplatin–paclitaxel | 32 | 18.8 | 4.2 | 14.9 | |||
| Sandler et al., 200611 (ECOG 4599) | Phase III | Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 434 | 35 | 6.2 | 12.3 | Not assessed. |
| Carboplatin–paclitaxel | 444 | 15 p<0.001 | 4.5 HR:0.66; 95% CI: 0.57 to 0.77 |
10.3 HR: 0.79; 95% CI: 0.67 to 0.92 |
|||
| Reck et al., 201012 (AVAiL) | Phase III | Cisplatin–gemcitabine with bevacizumab 15 mg/kg | 351 | 34.6 hr: 0.85; p=0.046 |
6.1 HR: 1.03; 95% CI: 0.86 to 1.23 |
13.4 | Not assessed. |
| Cisplatin–gemcitabine with bevacizumab 7.5 mg/kg | 345 | 37.8 hr: 0.75; p=0.0003 |
6.5 HR: 0.93 95% CI: 0.78 to 1.11 |
13.6 | |||
| Cisplatin–gemcitabine with placebo | 347 | 21.6 | 6.7 | 13.1 | |||
| Niho et al., 201213 (JO19907) | Randomized phase II |
Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 121 | 60.7 | 6.9 | 22.8 | Not assessed. |
| Carboplatin–paclitaxel | 59 | 31 | 5.9 HR: 0.61; 95% CI: 0.42 to 0.89 |
23.4 HR: 0.99; 95% CI: 0.65 to 1.50 |
|||
| Patel et al., 201314 (PointBreak) | Phase III | Carboplatin–pemetrexed with bevacizumab 15 mg/kg, followed by bevacizumab–pemetrexed | 472 | 34 | 6.0 | 12.6 | Not assessed. |
| Carboplatin–paclitaxel with bevacizumab 15 mg/kg, followed by bevacizumab | 467 | 33 | 5.6 HR: 0.83; 95% CI: 0.71 to 0.96 |
13.4 HR: 1.0; 95% CI: 0.86 to 1.16 |
|||
| Barlesi et al., 201415 (AVAPERL) | Phase III | Cisplatin–pemetrexed with bevacizumab 7.5 mg/kg, then randomized to maintenance | 376 | 22.7 | EORTC QLQ-30 and -LC13 identified no difference in global quality of life. Role function, fatigue, and appetite favoured bevacizumab. Pain favoured bevacizumab–pemetrexed. |
||
| Bevacizumab 7.5 mg with pemetrexed | 128 | 7.4 | 17.1 | ||||
| Bevacizumab 7.5 mg | 125 | 3.7 HR: 0.48; 95% CI: 0.35 to 0.66 |
13.2 HR: 0.87; 95% CI: 0.63 to 1.21 |
||||
| Seto et al., 201416 | Randomized phase II |
Erlotinib | 77 | 63.6 | 9.7 | Not reported | |
| Erlotinib–bevacizumab | 77 | 69.3 | 16 HR: 0.54; 95% CI: 0.36 to 0.79 |
||||
| Zhou et al., 201517 (BEYOND) | Phase III | Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 138 | 54 | 9.2 | 24.3 | Not assessed. |
| Carboplatin–paclitaxel with placebo | 138 | 26 | 6.5 HR: 0.40; 95% CI: 0.29 to 0.54 |
17.7 HR: 0.68; 95% CI: 0.50 to 0.93 |
|||
| Zinner et al., 201518 (PRONOUNCE) | Phase III | Carboplatin–pemetrexed, followed by pemetrexed | 182 | 23.6 | 3.9a | 10.5 | Not assessed. |
| Carboplatin–paclitaxel with bevacizumab 15 mg/kg, followed by bevacizumab | 179 | 27.4 | 2.9a HR: 0.85; 90% CI: 0.70 to 1.04 | 11.7 HR: 1.07; 95% CI: 0.83 to 1.36 |
|||
| Ramucirumab | |||||||
| Camidge et al., 201419 | Phase II | Carboplatin–paclitaxel with ramucirumab | 41 | 55 | 7.8 95% CI: 5.5 to 9.86 |
16.8 95% CI: 14.8 to 28.6 |
Not assessed. |
| Doebele et al., 201520 | Randomized phase II |
Cisplatin– or carboplatin–pemetrexed with ramucirumab 10 mg/kg, followed by pemetrexed–ramucirumab | 69 | 49.3 | 7.2 HR: 0.75; 90% CI: 0.55 to 1.03 |
13.9 HR: 0.83; 95% CI: 0.56 to 1.22 |
Not assessed. |
| Cisplatin– or carboplatin–pemetrexed followed by pemetrexed | 71 | 38 | 5.6 | 10.4 | |||
| Aflibercept | |||||||
| Chen et al., 201421 | Phase II | Cisplatin–pemetrexed–aflibercept | 42 | 26.3 | 5 95% CI: 4.3 to 7.1 |
NR | Trial stopped early because of 3 cases of reversible posterior leucoencephalopathy syndrome. |
Progression-free survival with no grade 4 toxicity.
Pts = patients; ORR = objective response rate; PFS = progression-free survival; OS = overall survival; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; CI = confidence interval; EORTC = European Organisation for Research and Treatment of Cancer; QLQ-30 = Quality of Life Questionnaire; LC13 = Lung Cancer module.