Introduction
Superior vena cava (SVC) syndrome occurs because of SVC obstruction; the obstruction can be due to external pressure on the vein or to an internal obstruction with thrombus formation in the vein.1, 2 With the growing use of intravenous catheters and other devices, benign etiologies of SVC syndrome have become more common.3, 4 Most etiologies of SVC syndrome are malignant with the most common being lung cancer, lymphomas (Hodgkin's and non-Hodgkin), and breast cancer.1, 2 Other benign causes include thyroid goiter, aortic aneurysm, tuberculosis and thymoma.2 In this article, we present the case of a woman with a malignant cause of SVC thrombosis but without any mass identified in the mediastinum.
Case report
A 46-year-old woman was admitted into hospital because of dyspnea, right arm pain and dysphagia. Forty-five days before admission she had developed pain in the right trunk and right scapula. Fifteen days prior to admission, she developed periorbital edema that slowly progressed to the entire face (Figure 1), neck, upper right extremity, upper trunk and both breasts. She also complained of erythema and pruritus of her forehead, cheeks and skin of the upper trunk during this period. She had evolved with progressive dyspnea and dysphagia for one week preceding admission. She had no history of fever, weight loss or night sweating. In the physical examination, the patient was not febrile and her respiratory rate was 22 breaths per minute. Beside the edema, collateral vessels were visible in her upper chest.
Figure 1.
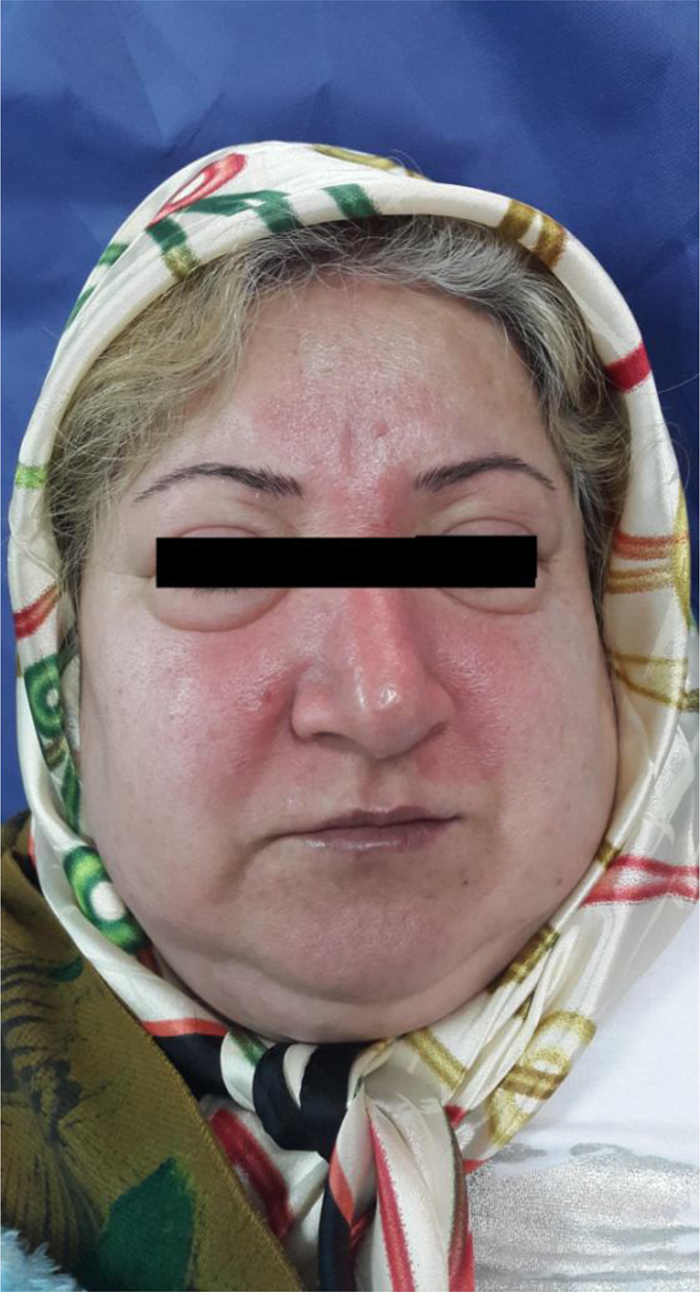
Face edema.
A chest X-ray was normal. Color Doppler ultrasonography of the upper extremity veins revealed normal flow in the left jugular vein, non-obstructive echogenic thrombosis in the right jugular vein and obstructive thrombosis in the right subclavian vein. A spiral chest computed tomography scan with contrast enhancement revealed bilateral pleural effusion in particular on the right side; thrombosis was identified in the superior vena cava that extended to the left subclavian vein. Moreover, bilateral lung nodules were found compatible with metastasis, as was a mass in the right rotator cuff muscles with scapular bone destruction (Figure 2). Laboratory data of the patient are shown in Table 1.
Figure 2.
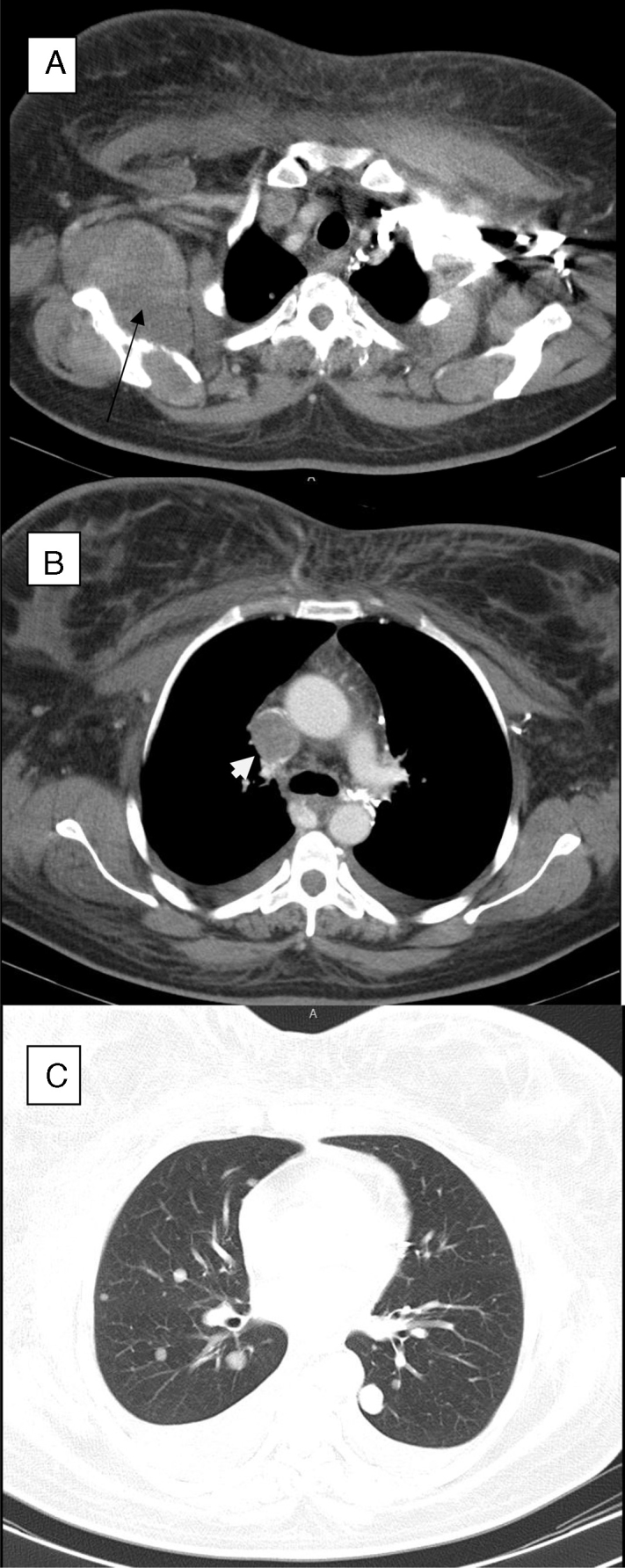
(A) Right rotator cuff mass (arrow) with scapular bone destruction. (B) Superior vena cava thrombosis (arrow head). (C) Pulmonary nodules.
Table 1.
Laboratory tests of the patient.
| Variable | Value | Reference value | Variable | Value | Reference value |
|---|---|---|---|---|---|
| WBC (/μL) | 8.1 × 103 | 4–10 × 103 | TSH (IU/mL) | 2.8 | 0.34–4.25 |
| Hemoglobin (g/dL) | 13 | 12.0–15.8 | PT (S) | 15 | 12–14 |
| Platelet (/L) | 306 × 109 | 165–415 × 109 | PTT (S) | 27 | 25–35 |
| Cr (mg/dL) | 1.0 | Female 0.5–0.9 | Sodium (meq/L) | 133 | 135–145 |
| Calcium (mg/dL) | 9.0 | 8.5–10.2 | Potassium (meq/L) | 4.0 | 3.5–5.5 |
| Alp (U/L) | Normal | Blood sugar (mg/dL) | 87 | 70–110 | |
| ESR (mm/h) | 25 | 0–20 | LDH (U/L) | 799 | (Upper-limit = 480) |
| CRP (mg/L) | 36 | <10 |
WBC, White blood cell count; Cr, Creatinine; Alp, Alkaline phosphatase; ESR, Erythrocyte sedimentation rate; CRP, C-reactive protein; TSH, Thyroid-stimulating hormone; PT, Prothrombin time; PTT, Partial thromboplastin time; LDH, Lactate dehydrogenase.
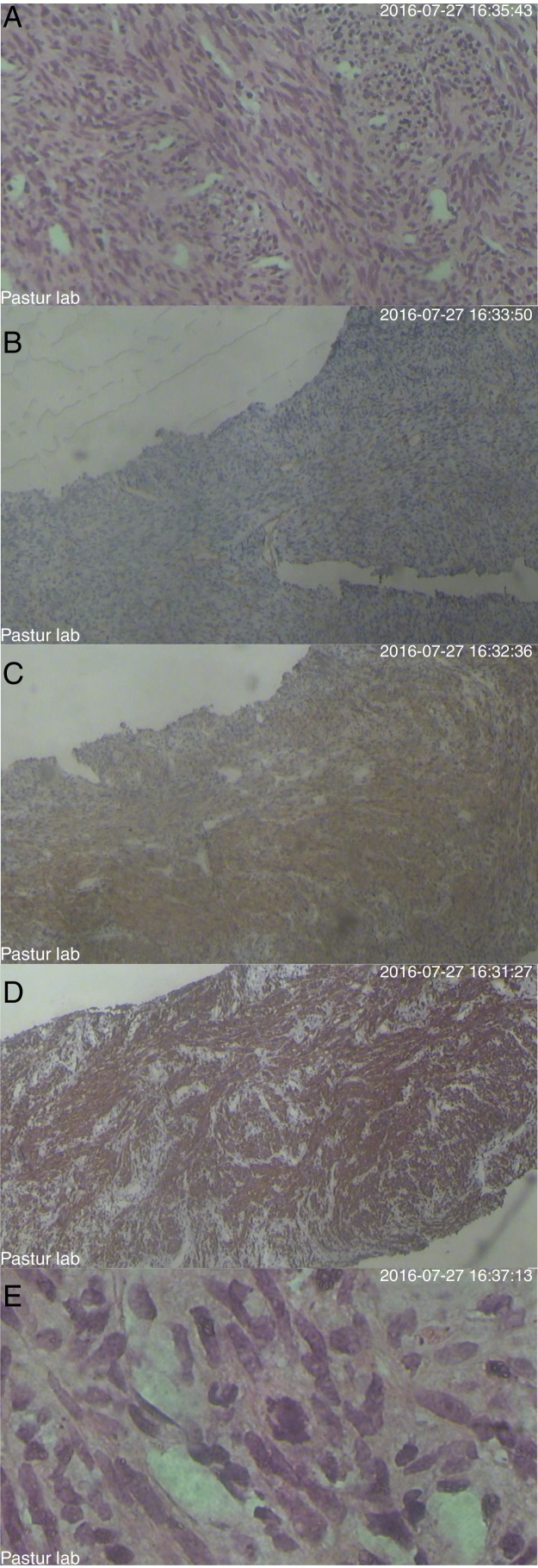
Enoxaparin therapy was initiated and core needle biopsy of the mass was performed. The pathology exam identified sarcoma which was confirmed by immunohistochemistry staining (Figure 3). Fifteen days after starting chemotherapy, her upper trunk edema was reduced significantly but the patient suddenly developed dyspnea and expired before any diagnostic procedure was performed. Pulmonary embolism was the most probable cause in spite of the regular enoxaparin injections from the time of her admission.
Figure 3.
Histology and immunohistochemistry of the tumor. (A) Wright's and Giemsa staining. (B) CD34 staining. (C) Smooth muscle actin (SMA). (D) Desmin. (E) Ki-67 protein.
Discussion
Intraluminal obstruction of the SVC may have benign or malignant causes. Long-term indwelling catheters, pacemaker wires and other intraluminal devices are common benign causes of SVC thrombosis.1, 2, 3, 4
Because the contrast enhanced computed tomography scan of our patient did not identify any mass, it follows that the SVC syndrome was a paraneoplastic syndrome. Santra et al. reported paraneoplastic thrombus formation in SVC due to lung cancer.5 Moreover, paraneoplastic causes of SVC syndrome due to thrombosis have been reported in association with renal cell carcinoma,6 ovarian cancer7 and Richter syndrome.8 Obstruction of the SVC can also be due to intraluminal metastasis without thrombus formation. Takayoshi et al. reported a case of adenosquamous carcinoma of the duodenum with intraluminal SVC metastasis.9 Furthermore, invasive thymoma10 and prostate cancer11 with intravenous metastasis have been reported. Thus, when a cancer is diagnosed but without any mass being identified in the upper mediastinum, paraneoplastic thrombosis is the most probable cause of SVC syndrome as in our case unless anticoagulation was ineffective for which a biopsy of the intravenous lesion is necessary. In addition, when a cause for SVC syndrome is not found, a biopsy is necessary for differential diagnosis, which includes metastasis, thrombosis, granuloma, fungal lesion, etc.
Most common symptoms are shortness of breath, cough, and swelling of face, neck, upper chest and extremities. Swelling may result in stridor, dysphagia and hoarseness. Chronic SVC syndrome causes distention of collateral veins, which may be seen in the upper chest.12 Pleural effusion is observed in 60% of cases.2 The signs and symptoms of our patient were compatible with chronic SVC syndrome.
Management includes chemotherapy or radiotherapy for malignant causes when a mass is the cause. Stent placement is increasingly being used to ameliorate compression of the vessel resulting from malignant causes.1, 2 Occasionally bypass surgery is performed.13 Other nonspecific methods include head elevation, mild diuresis and corticosteroids to decrease swelling and dyspnea. When thrombosis is the cause of SVC syndrome, thrombolysis1 and/or anticoagulation1, 2, 3, 4, 5 may be indicated. Our patient received anticoagulation and chemotherapy. Acute dyspnea and death were most probably due to pulmonary emboli. In the study of Paolo et al. of 842 patients with deep vein thrombosis, 181 were known to have cancer and recurrent thromboembolism occurred in 20.7%, most commonly during the first month of anticoagulation as in our patient.14
Conclusion
We reported an uncommon cause of SVC syndrome due to paraneoplastic SVC thrombosis, with a poor outcome, most probably due to pulmonary embolism during anticoagulation. Physicians should be alert during the management of SVC syndrome, in particular when the cause is malignant.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
We thank the Clinical Research development unit of Rouhani Hospital of Babol.
References
- 1.2015. Superior Vena Cava Syndrome/Approved by the Cancer.Net Editorial Board. Available from: http://www.cancer.net/navigating-cancer-care/side-effects/superior-vena-cava-syndrome [cited 23.11.17] [Google Scholar]
- 2.Cohen R., Mena D., Carbajal-Mendoza R., Matos N., Karki N. Superior vena cava syndrome: a medical emergency? Int J Angiol. 2008;17(1):43–46. doi: 10.1055/s-0031-1278280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garwood S., Flume P.A., Ravenel J. Superior vena cava syndrome related to indwelling intravenous catheters in patients with cystic fibrosis. Pediatr Pulmonol. 2006;41(7):683–687. doi: 10.1002/ppul.20388. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A.K., Khabiri H., Haddad N.J. Complications of vascular access: superior vena cava syndrome. Am J Kidney Dis. 2017;69(2):309–313. doi: 10.1053/j.ajkd.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Takayoshi K., Ariyama H., Tamura S., Yoda S., Arita T., Yamaguchi T. Intraluminal superior vena cava metastasis from adenosquamous carcinoma of the duodenum: a case report. Oncol Lett. 2016;11(1):605–609. doi: 10.3892/ol.2015.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May M., Seehafer M., Helke C., Uberrück T., Gunia S., Hoschke B. Superior vena cava syndrome with bilateral jugular and subclavian vein thrombosis. Paraneoplastic manifestion of renal cell carcinoma. Urologe A. 2003;42(10):1374–1377. doi: 10.1007/s00120-003-0401-9. [DOI] [PubMed] [Google Scholar]
- 7.Padovani M., Tillie-Leblond I., Vennin P., Demarcq G., Wallaert B. Paraneoplastic superior vena cava thrombosis disclosing an ovarian tumor. Rev Mal Respir. 1996;13(6):598–600. [PubMed] [Google Scholar]
- 8.Igala M., Kentos A., Dehou M.F., Unger P., Stoupel E., Bron D. Cardiac arrhythmias and superior vena cava syndrome revealing a Richter's syndrome. Rev Med Brux. 2014;35(2):69–71. [PubMed] [Google Scholar]
- 9.Santra A., Nandi S., Mondal S., Chakraborty S. Superior vena cava syndrome due to thrombosis: a rare paraneoplastic presentation of bronchogenic carcinoma. Iran J Med Sci. 2016;41(4):354–358. [PMC free article] [PubMed] [Google Scholar]
- 10.Panda P.K., Wig N., Kumar S., Arava S. Invasive thymoma presenting as classic superior vena cava syndrome: a case of venous spread metastasis. BMJ Case Rep. 2016;26 doi: 10.1136/bcr-2016-217695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda T., Saitoh M., Takeda S. Superior vena cava syndrome caused by an intravascular thrombosis due to underlying prostate carcinoma. Intern Med. 2008;47(22):2007–2009. doi: 10.2169/internalmedicine.47.1428. [DOI] [PubMed] [Google Scholar]
- 12.Wilson L.D., Detterbeck F.C., Yahalom J. Clinical practice, superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356(18):1862–1869. doi: 10.1056/NEJMcp067190. [DOI] [PubMed] [Google Scholar]
- 13.Gallo M., Protos A.N., Trivedi J.R., Slaughter M.S. Surgical treatment of benign superior vena cava syndrome. Ann Thorac Surg. 2016;102(4):e369–e371. doi: 10.1016/j.athoracsur.2016.03.112. [DOI] [PubMed] [Google Scholar]
- 14.Prandoni P., Lensing A.W., Piccioli A., Bernardi E., Simioni P., Girolami B. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]